Optical Biosensor Rapidly Detects Monkeypox Virus at Point of Care
Posted on 18 Nov 2024
A new variant of human mpox has caused a mortality rate of approximately 5% among those infected in the Democratic Republic of the Congo since 2023, with many of the victims being children. This variant has since spread to multiple other countries. On August 14, the World Health Organization declared the outbreak a Public Health Emergency of International Concern. In addition, another variant of mpox, which is less fatal, triggered an outbreak that has now spread to more than 100 countries since 2022. The symptoms of mpox, such as fever, pain, rashes, and lesions, closely resemble those of other viral infections, making it difficult for clinicians to differentiate monkeypox from similar diseases just by observation. Currently, polymerase chain reaction (PCR) is the only approved diagnostic method for mpox. However, PCR is costly, requires a lab, and can take several days or even weeks to return results. Therefore, there is a pressing need for more efficient and cost-effective diagnostic tools to control the spread of mpox and prepare for potential future pandemics.
Researchers from the University of California San Diego School of Medicine (La Jolla, CA, USA), Boston University (Boston, MA, USA), and their colleagues have developed an optical biosensor capable of rapidly detecting monkeypox, the virus responsible for mpox. This innovative technology could enable clinicians to diagnose the disease at the point of care, eliminating the need to wait for lab results. In their study, the team utilized a digital detection platform called Pixel-Diversity interferometric reflectance imaging sensor (PD-IRIS) to identify the virus. They tested samples taken from the lesions of a patient with confirmed mpox by incubating them with monoclonal monkeypox antibodies that bind to the virus's surface proteins. This virus-antibody complex was then transferred into small chambers on silicon chips on the sensor’s surface, which were treated to fix these nanoparticles.
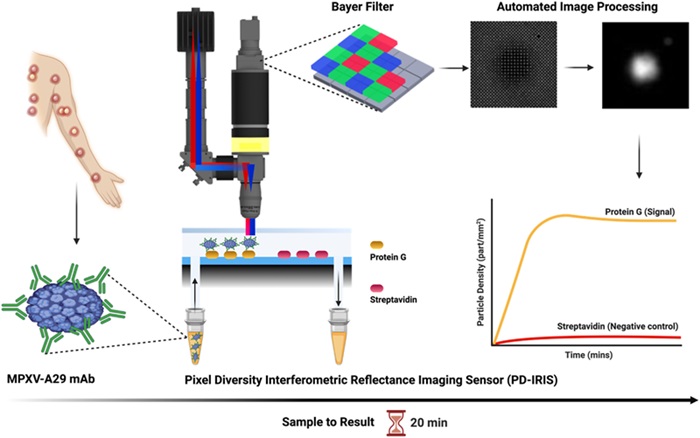
The sensor was activated by shining precise red and blue light wavelengths simultaneously on the chips, causing interference. This interference resulted in subtle variations in the response when the virus-antibody nanoparticles were present. A color camera captured this small signal and counted individual particles with high sensitivity. The team also analyzed samples from the herpes simplex virus and cowpox virus, which have similar clinical symptoms to mpox. The results, published in Biosensors and Bioelectronics, showed that the biosensor could effectively distinguish mpox from these other viruses, demonstrating its specificity in identifying monkeypox. Within two minutes, the test could confirm whether a patient has monkeypox, with the entire process, from sample collection to real-time data analysis, taking about 20 minutes.
In a clinical setting, the speed of this test would enable healthcare providers to diagnose mpox much more quickly than the traditional method of sending samples to a lab. This rapid diagnosis is especially crucial in regions with limited healthcare resources, as it can help curb the spread of the virus. Early diagnosis also allows clinicians to initiate treatment more promptly, if available. The researchers plan to mass-produce these tests in kit form for distribution to clinics, further reducing costs. A single kit could potentially test for multiple viruses, such as syphilis or HIV. The team aims to commercialize the technology, not only to address the urgent need for rapid mpox testing in the Democratic Republic of the Congo but also to prevent outbreaks from escalating into pandemics. However, the researchers emphasize that government support will be necessary to make these diagnostic tools available, as there is limited market interest in addressing future health threats.







 Analyzer.jpg)





