Mouth Swab Molecular Test for Tuberculosis Validated
By LabMedica International staff writers
Posted on 22 Jan 2020
Clinical investigators are testing an approach being developed to diagnose tuberculosis using mouth swabs, which could become an alternate approach to sample collection methods currently used to do tuberculosis (TB) testing.Posted on 22 Jan 2020
Partly because of the convenience of the collection method, the technique is helping increase access to testing and finding people with TB that have been undetected by traditional testing methods. For individuals with latent TB infection, the host maintains a dynamic relationship with Mycobacterium tuberculosis through the regulation of available nutrients as well as the innate and acquired immune systems.
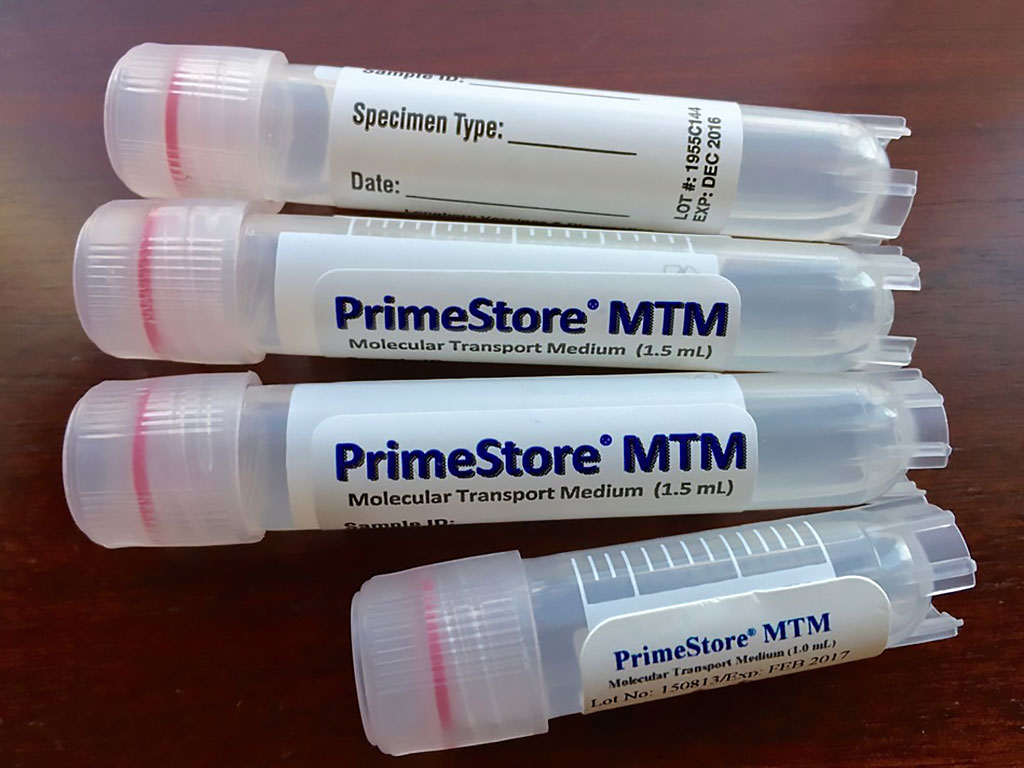
Image: PrimeStore Molecular Transport Medium (MTM) is the first molecular transport device designed to inactivate pathogens and stabilize both RNA and DNA for downstream molecular testing and characterization (Photo courtesy of Longhorn Vaccines and Diagnostics).
Scientists at the University of Pretoria (Pretoria, South Africa) and their colleagues collected and tested samples from people during house visits in an urban setting in South Africa with a high prevalence of TB and human immunodeficiency viruses (HIV). The team used flocked swabs to collect oral salivary specimens from 73 adults. Each person had a cough and one or more risk factors for TB, including previous TB, or was asymptomatic but residing with someone diagnosed with TB.
The investigators combined the swab samples that they had collected with PrimeStore Molecular Transport Medium (MTM, Longhorn Vaccines and Diagnostics, San Antonio, TX, USA) to enable microbial inactivation, DNA stabilization, and transportation of the specimens at ambient temperature to a nearby laboratory. The group detected M. tuberculosis DNA in 24, or 32.9%, of the 73 samples using Longhorn's polymerase chain reaction (PCR) diagnostic assay running on a Roche LightCycler platform (Roche Diagnostics, Basel, Switzerland).
P. Bernard Fourie, PhD, a professor of medical microbiology and TB specialist, said, “The approach holds promise as an easy to perform, safe, and patient-friendly procedure for triaging people who are presumed to have TB at the household level, and it provides a basis for targeted patient follow-up.”
Related Links:
University of Pretoria
Longhorn Vaccines and Diagnostics
Roche Diagnostics