Tests Links Circulating Tumor DNA and Breast Cancer Survival
By LabMedica International staff writers
Posted on 27 Feb 2018
Cancer researchers used a liquid biopsy test for circulating tumor DNA (ctDNA) to profile cancer genomes from blood samples and predict survival outcomes for patients with metastatic triple negative breast cancer.Posted on 27 Feb 2018
Triple-negative breast cancer (TNBC) refers to any breast cancer that does not express the genes for estrogen receptor (ER), progesterone receptor (PR), and Her2/neu. While TNBC represents just 10-15% of all breast cancer diagnoses, the disease is responsible for 35% of all breast cancer-related deaths. TNBC is characterized by few mutations but displays extensive somatic copy number alterations (SCNAs). However, little is known regarding SCNAs in metastatic TNBC.
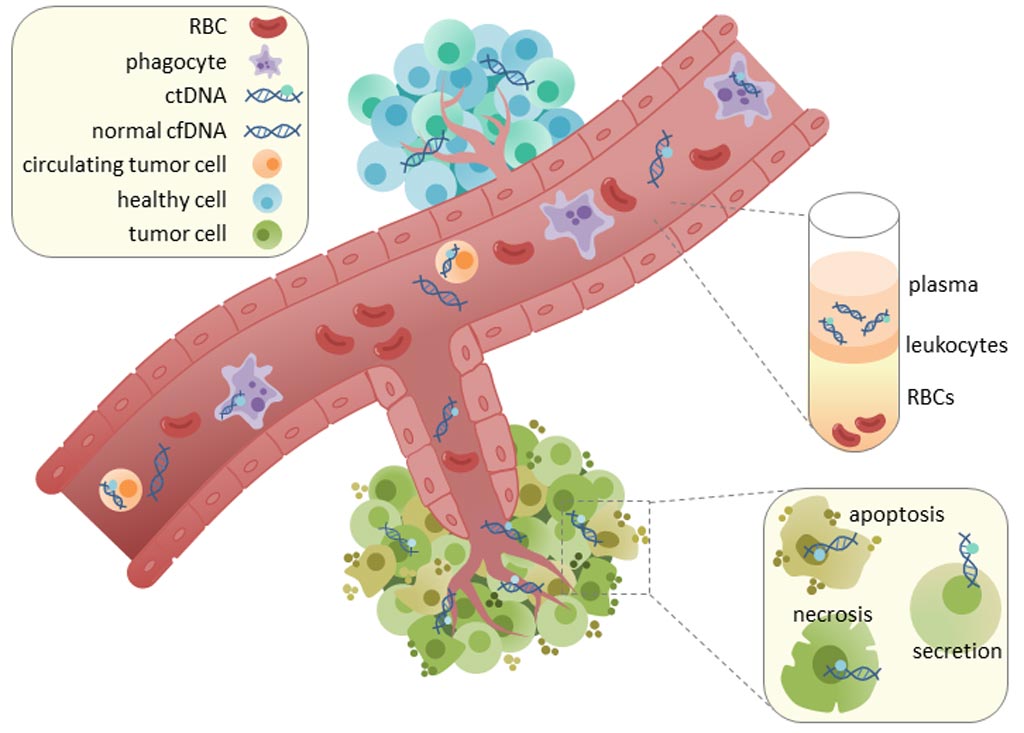
Image: Circulating tumor DNA (ctDNA) is found in serum and plasma fractions from blood. The mechanism of ctDNA release is unknown, though apoptosis, necrosis, and active secretion from tumor cells have been hypothesized (Photo courtesy of Wikimedia Commons).
A team of investigators from several research institutes sought to evaluate SCNAs in metastatic TNBC exclusively via ctDNA and determine if ctDNA tumor fraction was associated with overall survival in metastatic TNBC. To this end, the investigators identified 164 patients with biopsy-proven metastatic TNBC at a single tertiary care institution who had received prior chemotherapy and performed low-coverage genome-wide sequencing of ctDNA from their plasma.
Results revealed that without prior knowledge of tumor mutations, the investigators determined tumor fraction of ctDNA for 96.3% of patients and SCNAs for 63.9% of patients. Copy number profiles and percent genome altered were remarkably similar between metastatic and primary TNBCs. Certain SCNAs were more frequent in metastatic TNBCs relative to paired primary tumors and data for primary TNBCs published in publicly available data sets. Overall, 64% of patients had more than 10% tumor DNA, and that this threshold of tumor DNA was correlated with poor survival outcomes in patients with metastatic TNBC.
"The recognition that a significant fraction of patients harbor greater than 10% tumor DNA in blood suggests that liquid biopsies may enable routine and non-invasive profiling of cancer genomes for patients with metastatic TNBC," said senior author Dr. Viktor Adalsteinsson, group leader of the blood biopsy team at the Broad Institute (Cambridge, MA, USA).
The study was published in the February 20, 2018, issue of the Journal of Clinical Oncology.
Related Links:
Broad Institute













