Non-Invasive COVID-19 Diagnostic System Collects Sample Droplets from Lungs and Returns Results in 10 Minutes
By LabMedica International staff writers
Posted on 22 Jul 2021
A non-invasive exhaled breath diagnostic technology offers the potential for a simple, inexpensive, non-invasive, massively deployable, rapid diagnostic system for detecting respiratory illness and airborne viral threats, including COVID-19, in approximately 10 minutes.Posted on 22 Jul 2021
The non-invasive exhaled breath diagnostic technology, BlowFISH, developed by Marvel Diagnostics Inc. based at University of California (Los Angeles, CA, USA) and funded by Medivolve Inc. (Toronto, Canada) has successfully cleared the first milestone in a series of clinical tests targeting application of an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) to test for the COVID-19 virus. During the clinical trial, BlowFISH’s proprietary technology, designed to efficiently collect a substantial liquid sampling directly from deep within the lungs, successfully detected the COVID-19 virus in three test samples.
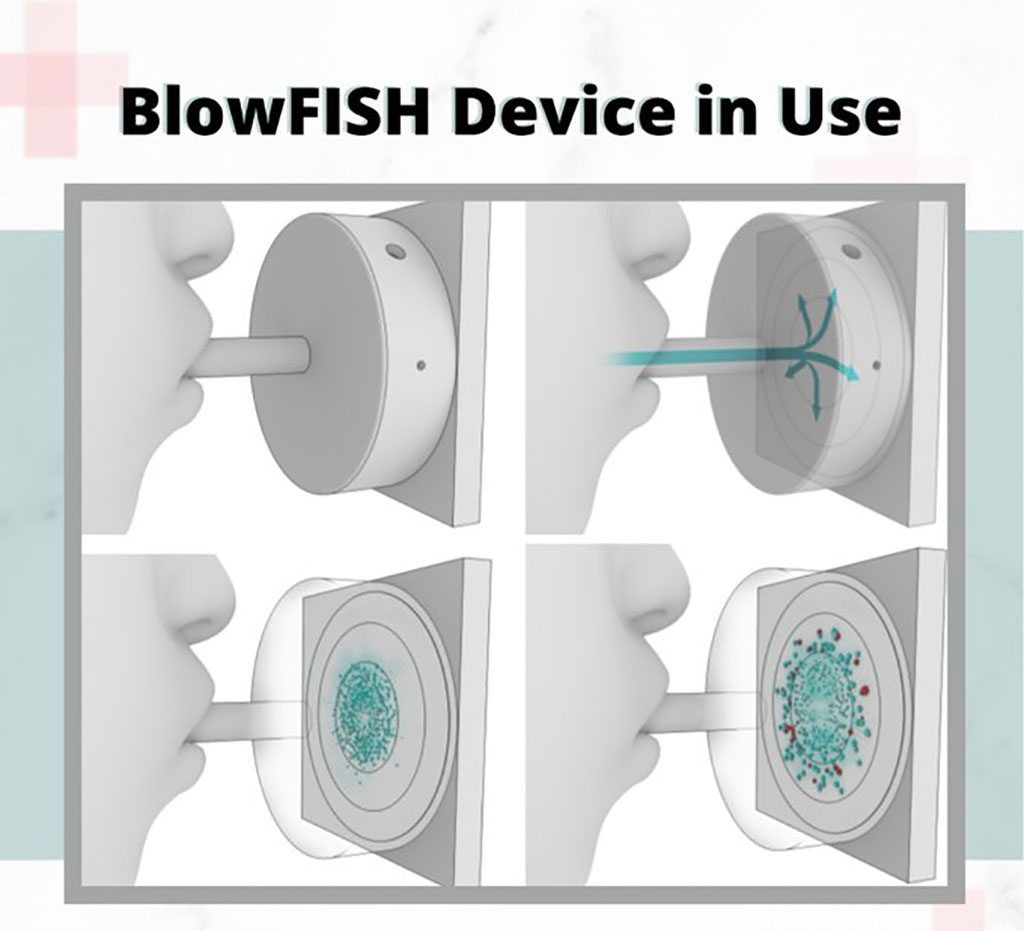
Image: Novel BlowFISH device that captures virus within Exhaled Breath Condensate (EBC) (Photo courtesy of Medivolve Inc.)
“This is an exciting and important milestone in advancing BlowFISH toward achieving EUA status in testing for COVID-19, and providing a non-invasive, cost-effective and scalable testing alternative to nasal swab solutions currently in market," said David Preiner, CEO, Medivolve. “Making testing more accessible to populations, such as children and the elderly, where it may be difficult to administer a nasopharyngeal swab test, will become important in our transition to resuming daily life in the 'new normal'.”
“BlowFISH’s detection of the COVID-19 virus brings us one significant step closer to changing the future of diagnostics for not only COVID-19, but for a wide range of respiratory illnesses,” said Dr. Pirouz Kavehpour, UCLA Professor and Marvel Diagnostics Co-Founder. “We are moving forward with urgency through proof-of-concept clinical trials, as these studies are a critical next step in making respiratory testing more comfortable, convenient and accessible for all...one breath at a time.”
Related Links:
Medivolve Inc.













