Immunoassays Could Predict Drug Failure in Arthritis Patients
By LabMedica International staff writers
Posted on 21 Jul 2015
Biologic drugs have dramatically improved the long-term health of people with severe rheumatoid arthritis (RA) reducing symptoms as well as joint damage and disability, but in about one in five patients the treatment stops working after a few months limiting their effectiveness.Posted on 21 Jul 2015
Biologics are a relatively new form of treatment for RA and are given by injection and they work by stopping particular chemicals in the blood from activating the immune system and attacking the joints. Biologics are usually given in combination with an anti-rheumatic once the anti-rheumatic alone is no longer effective.
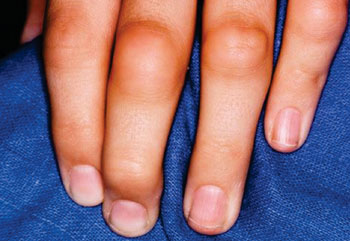
Image: Joint inflammation from rheumatoid arthritis is typically symmetrical, occurring on both sides of the body at the same time (Photo courtesy of Dr. Melinda Ratini).
Scientists at The University of Manchester (UK) and their colleagues studied 311 patients in order to predict early which rheumatoid arthritis (RA) patients will fail to respond to the biologic drugs given to treat them. These findings could help better manage patients' symptoms. At baseline and following initiation of therapy, patients had serum samples collected with disease activity measured at 3, 6, and 12 months.
Serum drug levels were tested in all serial samples after initiation of treatment and were measured in-house using a sandwich enzyme-linked immunosorbent assay (ELISA) (Progenika Biopharma; Derio, Spain). The presence of anti-drug antibodies (ADAbs) to adalimumab and etanercept were determined using radioimmunoassay (RIA) (Sanquin Diagnostic Services; Amsterdam, Netherlands). The assay measures specific high-avidity immunoglobulin G (IgG) antibodies against the drug by an antigen-binding test.
The scientists revealed that a total of 25% of patients on adalimumab developed antibodies, but none were found in the patients using etanercept. They also found that higher doses of methotrexate, a drug often given together with the biologic treatment, was associated with lower levels of drug antibodies, suggesting that patients should be encouraged to continue methotrexate at the highest dose they can tolerate, to reduce the risk of developing anti-drug antibodies.
Meghna Jani, MBChB, MSc, MRCP, the lead author of the study, said, “Our study demonstrates detecting low drug levels in rheumatoid arthritis patients on adalimumab, one of the most commonly prescribed biologics, was the strongest factor associated with non-response to treatment over 12 months. This test is easy to perform in a hospital setting, and could provide useful information on how to manage a patient whose rheumatoid arthritis is not being controlled by adalimumab.” The study was published online on June 24, 2015, in the journal Arthritis & Rheumatology.
Related Links:
The University of Manchester
Progenika Biopharma
Sanquin Diagnostic Services













