New Blood Draw Device Evaluated For Hemolysis
By LabMedica International staff writers
Posted on 06 Feb 2018
Posted on 06 Feb 2018
Hemolysis, defined as the breakdown of red blood cells and the release of hemoglobin and intracellular contents into the plasma, is a frequent occurrence in blood samples submitted to clinical laboratories for testing.
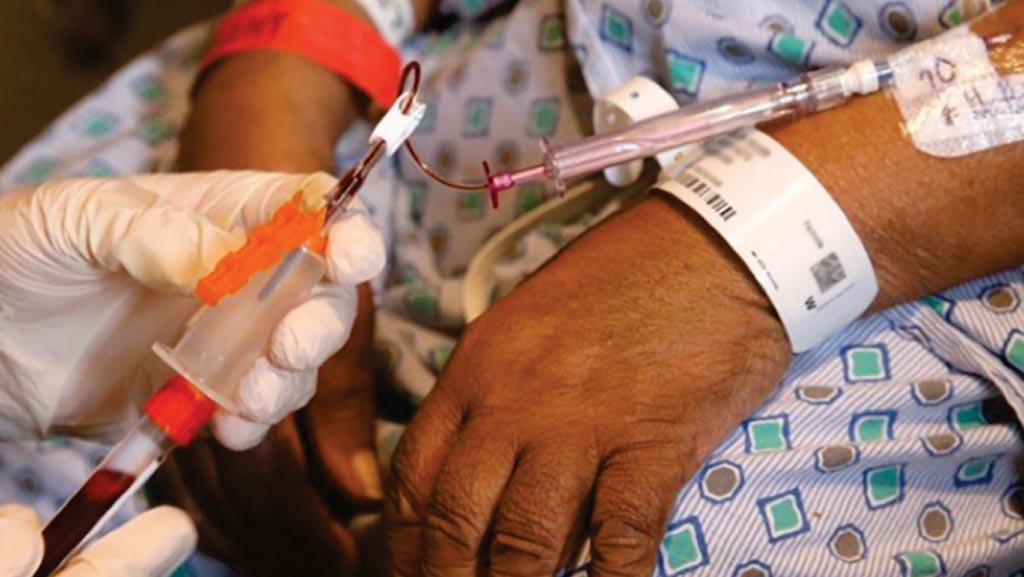
Image: A blood draw using the PIVO device which allows patients to have blood taken multiple times during their hospital stay without having to undergo multiple needle sticks (Photo courtesy of Intermountain Healthcare).
Blood collections from peripheral intravenous catheters offer several benefits to patients, including reduced needle punctures and patient discomfort, but they risk reducing the quality of blood specimens analyzed by the laboratory. The estimated prevalence of hemolyzed specimens is approximately 3% of routine samples and they make up approximately 60% of specimens classified as unsuitable for analysis.
A team of medical laboratory scientists led by those at University Hospitals Cleveland Medical Center (Cleveland, OH, USA), in an effort to balance analytical quality of test results with patient-centered care initiatives, a needle-less blood collection device called PIVO (Velano Vascular, San Francisco, CA, USA) was evaluated at two institutions. The primary objective of this study was to assess the ability of the PIVO device to provide high-quality blood specimens for laboratory testing compared to current blood collection methods.
The PIVO blood collection device was used for blood draws from a peripheral intravenous (IV) catheter (PIVC). Prior to the PIVO collection, the IV catheter was flushed with 5 mL normal saline. The PIVO device was attached to the needle-less valve and actuated through the IV catheter into the blood stream. Standard vacuum tubes or a syringe were used at the back end of the device to collect blood samples. The blood collections were typically performed with a tourniquet above the PIVC. A discard volume of 1 mL was collected through PIVO prior to the required specimen collection. After the PIVO collection the device was retracted, removed, and disposed of. The IV catheter was again flushed with 5 mL normal saline.
Specimen integrity was checked by the semi-quantitative, spectrophotometric assessment of hemolysis in human serum and plasma on automated chemistry analyzers. Hemolysis flags were appended to test results when the hemolysis index corresponding to a free hemoglobin concentration of ≥ 50 mg/dL was triggered for blood chemistry test samples requiring an automated specimen integrity check. Approximately 7,600 PIVO blood draws were performed across the two institutions. The hemolysis rates of samples collected with PIVO were evaluated using 2,380 flagged collections, containing approximately 1,200 test orders requiring hemolysis index measurements. The hemolysis rate of PIVO-flagged samples (1.8%) was statistically superior to the venipuncture and central line blood collection methods (3.3%), reducing the risk of hemolysis during a venous blood draw by 39%.
The authors concluded that PIVO collections facilitated improvement in the rate and degree of sample hemolysis when compared to venipuncture and central line blood collections. These findings suggest that PIVO is capable of delivering samples that are superior to current blood collection methods in terms of hemolysis rate as well as reducing the number of invasive venipunctures required for laboratory testing. The study was published on January 4, 2018, in the journal Practical Laboratory Medicine.













