DNA Test Launched for Laser Eye Surgery Safety
By LabMedica International staff writers
Posted on 27 May 2014
A genetic test has been introduced that can detect both Avellino Corneal Dystrophy (ACD) and another genetic mutation, Granular Corneal Dystrophy type I (GCD1).Posted on 27 May 2014
A patient with GCD1 who undergoes vision correction surgery, such as Laser-Assisted in situ Keratomileusis (LASIK), Laser-Assisted Sub-Epithelial Keratectomy (LASEK) or Photorefractive keratectomy (PRK) are at extreme risk of experiencing eventual blindness.
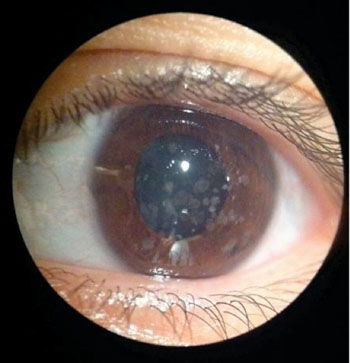
Image: Granular corneal dystrophy (Photo courtesy of Dr. B.H. Feldman).
The Avellino DNA Dual Test (Avellino Laboratory; Menlo Park, CA, USA) is able to detect the presence of the genetic mutation, allowing the patient to take precautionary steps to postpone the progression of the condition, including avoiding vision correction surgery. The Avellino DNA Dual Test is easy and safe. The test involves a simple mouth swab to determine whether a person carries the GCD1 or the GCD2 (ACD) gene mutation. Specifically, the ophthalmologist takes 10 swipes from the inside of each cheek in order to obtain an adequate sample. The sample is then sent to Avellino Lab USA, a Clinical Laboratory Improvement Amendments (CLIA) certified molecular diagnostic testing laboratory. Within 24 to 48 hours, the results are provided to the physician to share with the patient. Avellino Laboratory has branches in North America, Western Europe, and Asia.
Granular Corneal Dystrophy (type 1 and 2) has been diagnosed in patients throughout the world and is one of the more well-known corneal dystrophies related to genetic mutations. Unfortunately, many physicians assume incorrectly that they can diagnose the condition through a visual examination and family history. However, many patients do not show physical symptoms of the condition until later in life. Consequently, relying solely on traditional methods for diagnosis can put patients at risk.
Tom Tooma, MD, founder of NVISION Laser Eye Center, said, (Newport Beach, CA, USA) said, “We have been utilizing the Avellino DNA Test for LASIK Safety since its availability in the United States, and it has been a fantastic tool for both our physicians and patients in increasing their confidence prior to LASIK. Now, with the Avellino DNA Dual Test, patients can go into treatment knowing they are even more protected from adverse outcomes such as loss of vision.” The Avellino DNA Test for LASIK Safety has become the standard of care in Korea with 160 LASIK clinics using this test and in Japan more than 80% of LASIK patients were tested.
Related Links:
Avellino Laboratory
NVISION Laser Eye Center














