Biomarker Identified for Aggressive Thyroid Cancer
By LabMedica International staff writers
Posted on 25 Nov 2016
A biomarker has been identified for the aggressive disease of papillary thyroid cancer, which comprises about 90% of all thyroid cancers and treatment of the disease historically relied upon a combination of surgery and radioactive iodine ablation with few alternatives if the disease progresses.Posted on 25 Nov 2016
Each year approximately 6,300 Canadians will be diagnosed with thyroid cancer and more than three quarters of those patients are women. Treatments for the disease include radioactive iodine therapy and surgery and those who opt for aggressive surgery can see their speech affected, have trouble eating, swallowing and even breathing as a result.
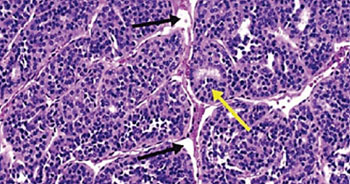
Image: A histopathology of insular thyroid carcinoma, showing the nesting patterns of the follicles (yellow arrow) and the artefactually created clefts (black arrows) (Photo courtesy of H. Lee Moffitt Cancer Center).
A team of scientists at the University of Alberta (Edmonton, Canada) selected a total of 287 patient specimens were with thyroid tumors of which 181 are papillary thyroid carcinomas (113 without and 68 with lymphatic metastases), 57 are benign follicular neoplasms and there are 36 normal thyroid tissue specimens and 13 sections of metastatic lymph nodes. Recurrence was defined as an increase in unstimulated thyroglobulin levels of greater than 0.4 ng/mL, stimulated thyroglobulin levels greater than 2 ng/mL, and/or pathologic evidence of recurrence based on ultrasound-guided fine needle aspiration biopsy.
Primary thyroid cancer cells were obtained using the Cancer Cell Isolation Kit (Panomics, Inc., Fremont, CA, USA). To study the platelet derived growth factor receptor alpha (PDGFRα) the scientists used stable transduction techniques, gene transfer, wound healing, clonogenic, transwell invasion and proliferation assays. Among other methodologies the team performed mouse xenografts, sodium iodide uptake and immunochemistry.
The investigators found that PDGFRα expression can be tested in tumor specimens to predict aggressive disease. In addition, they showed that targeting PDGFRα could restore sensitivity to radioactive iodine treatment that might slow disease growth and spread. Patients exhibiting PDGFRα at time of diagnosis are three times more likely to exhibit nodal metastases and are 18 times more likely to recur within five years than those patients lacking PDGFRα expression. Moreover, high levels of PDGFRα and low levels of nuclear transcriptional activity of thyroid transcription factor-1 (TTF1) predict resistance to radioactive iodine therapy.
Todd P.W. McMullen, MD, PhD, the senior author and associate professor of surgery, said, “We came up with a tool to identify aggressive tumors so that people can have just the right amount of surgery. No more, no less. What we're really excited about is that this is both a diagnostic tool and a therapy. It can be used to do both. We've identified the mechanism of how this protein actually drives metastasis in thyroid cancer. And not only that, we found out that it also makes the cancer resistant to radioactive iodine therapy.” The study was published in the October 2016 issue of the journal EbioMedicine.
Related Links:
University of Alberta
Cancer Cell Isolation Kit













