Equipment-Free Diagnostic Test Enables Detection of SARS-CoV-2 and Variants at POC
Posted on 17 Aug 2022
More than two years into the pandemic, the virus that causes COVID—SARS-CoV-2—continues to spread worldwide. Testing for the virus and its variants can help limit transmission and inform treatment decisions, and is therefore an important pillar of the public health response. Now, researchers have created an easy-to-use diagnostic test for COVID infection that is more sensitive than the commonly used at-home antigen tests, and that also allows for the rapid and specific detection of SARS-CoV-2 variants in point-of-care settings.
The improved test developed by a team of researchers from Princeton University (Princeton, NJ, USA) and the Broad Institute of MIT and Harvard (Cambridge, MA, USA) detects virus using a different mechanism than the more familiar clinic-based PCR or at-home antigen tests. Instead, the test detects virus using a technology known as CRISPR, which has found widespread use in gene editing. CRISPR originated from a system used by bacteria to detect and defend against viral infections, and is uniquely suited to rapidly identifying specific genetic sequences.
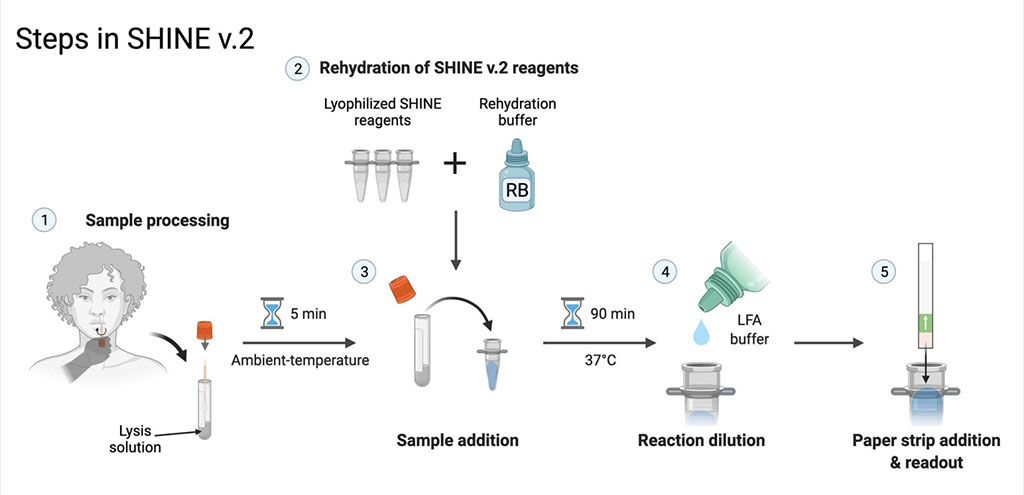
The researchers had earlier developed develop a CRISPR-based test that could be tailored to diagnose specific viral infections. In the wake of the pandemic’s first wave in 2020, the team modified their test, which they called SHINE, to detect SARS-CoV-2 with remarkable sensitivity. In a collaborative effort to improve SHINE, the group first focused on eliminating the need for specialized equipment to prepare patient samples for testing. Next, they optimized the test so that its reagents don’t need to be kept in a freezer, which ensures that the test can be easily transported long distances.
They dubbed the improved test “SHINEv.2.” After confirming that the altered test worked well in their own laboratory, the group sent a test kit to a laboratory in Nigeria to see whether it could survive a long period in transit and still retain its accuracy and sensitivity. It did. But then a new challenge arose for the team to address – the emergence of new SARS-CoV-2 variants. The team was able to quickly adapt SHINEv.2 to discern between infections mounted by the Alpha, Beta, Delta or Omicron SARS-CoV-2 variants in patient samples, and say the test can be rapidly modified to pick up on any other variants that may arise.
Because it tests for the different variants all at once, this version of the test needs one special, but relatively inexpensive, piece of equipment to read out its results. Nonetheless, SHINEv.2 is much faster to perform, and requires much less equipment and expertise, than current approaches used to identify SARS-CoV-2 variants. The team envisions that it would mainly be used at doctors’ offices, even ones with limited resources in remote locations, to help physicians determine which viral variant is afflicting their patient, and tailor therapies appropriately.
“The data indicating this approach can be used to distinguish SARS-CoV-2 variants of concern by differential detection of genomic mutations could enable us to better prepare for the appearance of the next mutation,” said Tony Hu, director of the Center for Intelligent Molecular Diagnostics and Weatherhead Presidential Chair at Tulane University, who reviewed the manuscript for Nature Biomedical Engineering. “The approach reported by authors could be a significant contribution for pandemic control by providing a method that requires minimal sample processing or heating.”
Related Links:
Princeton University
Broad Institute of MIT and Harvard














