Two-Minute COVID-19 Test Uses Nanosensor Technology to Detect Presence of SARS-CoV-2 Infection in Blood at POC
By LabMedica International staff writers
Posted on 16 Jul 2021
A SARS-CoV-2 testing system addresses existing diagnostic challenges by providing a rapid assessment from a nasal, throat, saliva, or finger stick of blood sample in a two minute test.Posted on 16 Jul 2021
The simple, low-cost system that includes a portable handheld, analyzer, disposable cartridges and secure software for seamless systems integration has been developed by NanoDx, Inc. (Boston, MA, USA). NanoDx is licensing IBM Research’s (Yorktown Heights, NY, USA) nanoscale sensor technology for use in its diagnostic platform. IBM’s technology was developed with the goal of advancing sensor metal-oxide semi-conductive (CMOS) technology.
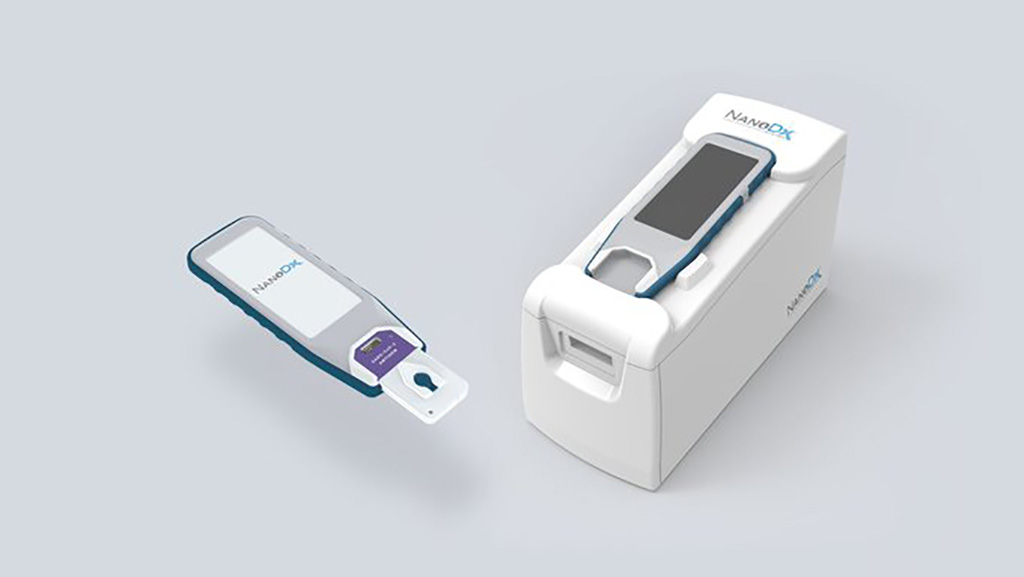
Image: Two-Minute COVID-19 Test Uses Nanosensor Technology to Detect Presence of SARS-CoV-2 Infection in Blood at POC (Photo courtesy of NanoDx, Inc.)
NanoDx plans to use this sensor technology for diagnostic platforms designed to provide rapid, accurate and inexpensive detection of different diseases. NanoDx also plans to use this technology to advance efforts to diagnose a variety of medical conditions rapidly and accurately, including COVID-19, different forms of influenza, traumatic brain injury (TBI), sepsis and stroke in the field of in vitro diagnostics, as well as biosensors. The IBM-designed nano biosensors are CMOS-compatible, which means they may be more cost-effectively and rapidly manufactured in high-volume. When integrated with automation circuitry, these tiny sensors may enable NanoDx’s real-time, point-of-care diagnostics technology to detect and quantify biomarkers from small fluid specimens in less than two minutes.
NanoDx is commercializing its real-time, point-of-care diagnostics platform and has built an extensive intellectual property portfolio around its nanosensor design and manufacturing processes that encompasses the entire field of in vitro diagnostics. The NanoDx System for SARS-CoV-2 is pursuing FDA Emergency Use Authorization to expand rapid COVID-19 testing.
"The field of diagnostics has changed rapidly during the past year with the emergence of COVID-19," said Sharad Joshi, President and Chief Executive Officer of NanoDx. "This global pandemic has further highlighted the need for a convenient, fast and accurate diagnostic so proper treatments can be delivered to patients as quickly as possible with the goal of shortening recovery times. As we move forward, our access to IBM's technology in the in vitro diagnostics and biosensor fields will enable us to pursue developing a high throughput diagnostics platform at a significantly lower cost."
"Our work with NanoDx is another example of IBM's collaborative approach to what we call 'hard tech': solving the deep technical problems that affect our everyday lives," said Mukesh Khare, Vice President, Hybrid Cloud Research at IBM Research. "Notably, this CMOS-compatible device can be manufactured in an existing foundry, providing easily scalable and cost-effective hardware. The integration of this technology design with NanoDx's highly-advanced nanosensor platform can help accelerate the ability to bring an extensive array of diagnostic solutions to market."
Related Links:
NanoDx, Inc.
IBM Research










 Analyzer.jpg)



