First-Ever Single-Use, PCR Quality OTC COVID-19 At-Home Test Granted FDA EUA
By LabMedica International staff writers
Posted on 15 Apr 2021
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the first-ever single use, over the counter (OTC) sale of a COVID-19 test kit that delivers PCR quality molecular accuracy in 30 minutes or less at home.Posted on 15 Apr 2021
Lucira Health, Inc. (Emeryville, CA, USA) has received EUA from the FDA for its LUCIRA CHECK IT test kit that is now authorized and available for individuals with or without COVID-19 symptoms. This primarily US designed and manufactured product is the first FDA authorized, prescription, molecular diagnostic test for COVID-19 that can be self-administered by patients at home or used in a physician’s office. OTC clearance dramatically expands the availability of this highly accurate test.
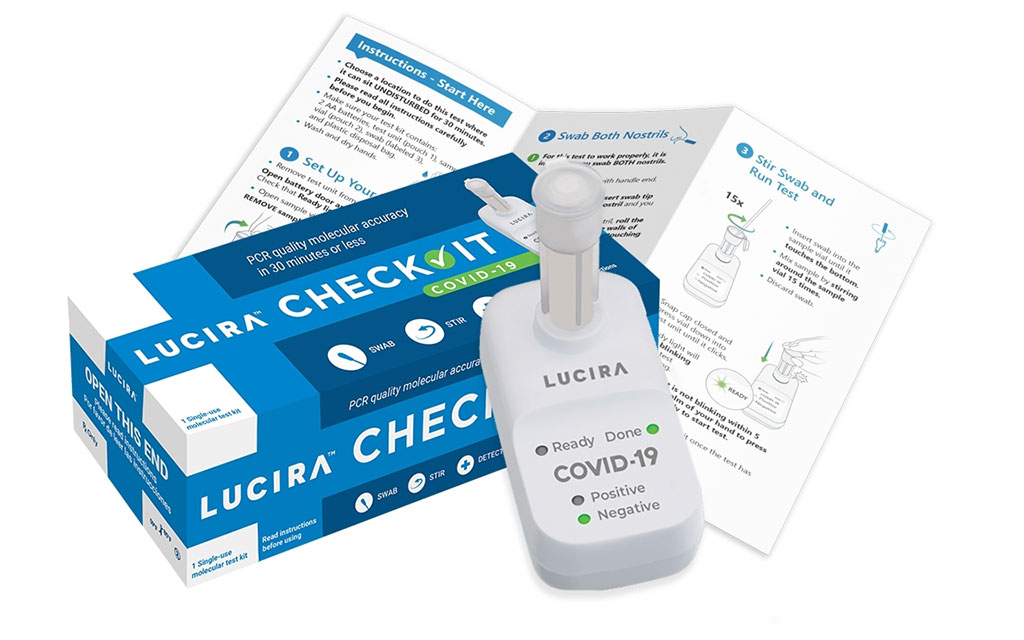
Image: The LUCIRA CHECK IT test kit (Photo courtesy of Lucira Health, Inc.)
The Lucira CHECK IT Test Kit fits in the palm of a hand, extracts genetic material from the virus and amplifies it similar to PCR lab tests. Each Lucira test kit contains everything needed to run one COVID-19 test. Users get the test device, two AA batteries, sample vial, swab and simple instructions. The batteries are inserted in the device and the sample vial is placed in the test unit. The user then opens the test swab packet and rotates the swab in each nostril five times. The swab is then stirred in the sample vial, which is then gently pressed into the test unit to start the test. The “ready” light will blink until a “positive” or “negative” green light is illuminated within 30 minutes. It can detect a positive result in as few as 11 minutes or confirm a negative result within 30 minutes.
In clinical trials, Lucira’s easy-to-use ‘swab, stir and detect’ CHECK IT test kit demonstrated that 100% of users successfully performed the test in about two minutes. Labs currently take two to 14 days to generate similarly accurate test results. Molecular tests are more sensitive than antigen tests because they amplify critical parts of the viral target. The targeted, molecular amplification that Lucira CHECK IT and PCR tests employ makes them demonstrably more sensitive and reliable than “rapid” antigen tests, which can miss active COVID-19 infections.
To support people who would like to quickly receive confirmation of their test results for work and other needs, Lucira offers a secure, text-based way for people with smartphones to receive a free LUCI Pass without downloading an app. The LUCI Pass was developed to support Lucira’s OTC test kit and is unique to Lucira. Users simply text a short code to access LUCI, and then go through a simple sequence of steps including scanning their test result to receive a LUCI Pass and verified test to their phone. Results are also transmitted to the required public health authorities.
“We worked with more than 1,000 people before starting our FDA clinical and usability studies. Our goal was to produce an easy-to-use test that provides PCR quality accuracy in a portable, intuitive, anytime, anywhere format,” said Lucira CEO Erik Engelson. “People are looking for ways to feel more certain in these uncertain times, and our Lucira CHECK IT test provides that.”
Related Links:
Lucira Health, Inc.













