Rapid Decentralized Testing for Diagnosis of COVID-19
By LabMedica International staff writers
Posted on 15 Dec 2020
A novel "nanoPCR" technique enables diagnosis of COVID-19 infections in less than 20 minutes with the accuracy of the current PCR method.Posted on 15 Dec 2020
The diagnosis of COVID-19 by quantitative PCR with reverse transcription (RT–qPCR) typically involves bulky instrumentation in centralized laboratories and an assay time of one to two hours. The logistics process of cold chain transportation from the sampling sites to the testing facility slows conventional RT-PCR diagnosis even more, often taking one to two days to yield results.
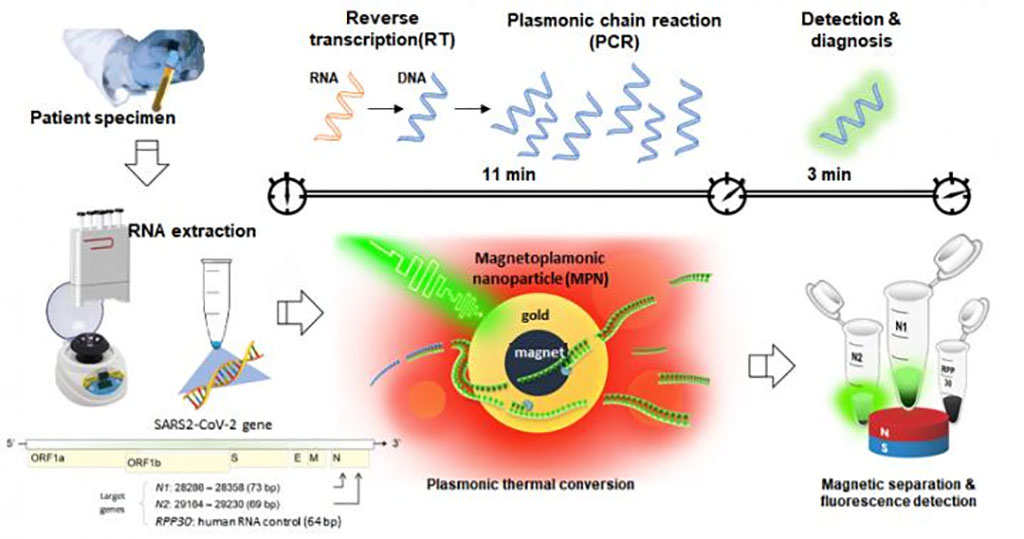
Image: The RNA of the coronavirus was extracted from the patient sample, which then underwent reverse transcription, gene amplification, and detection through nanoPCR to diagnose the COVID-19 infection. For rapid gene amplification and detection, magneto-plasmonic nanoparticles (MPNs) were used to facilitate the temperature change cycle of the existing RT-PCR at high speed. Finally, a magnetic field separated the MPN, and the fluorescent signal of the amplified DNA was detected (Photo courtesy of Institute for Basic Science, Korea)
To improve this situation and provide a platform for decentralized testing of suspected COVID-19 patients, investigators at the Institute for Basic Science (Seoul, South Korea) and colleagues in Korea and the United States developed “nanoPCR”, a fast one-pot PCR with reverse transcription (RT–PCR) technology.
The nanoPCR system seamlessly integrated plasmonic thermocycling with fluorescent signal detection in a single device. A key concept was the use of dual-functional magneto-plasmonic nanoparticles (MPNs) for PCR applications. The investigators noticed that most of the plasmonic effect was confined near the surface of plasmonic nanoparticles. By encasing a magnetic core with a plasmonic gold (Au) shell, they achieved: (1) efficient plasmonic heating comparable to that by solid Au nanoparticles; and (2) rapid nanoparticle separation with external magnetic fields to perform in situ signal detection. Exploiting these advantages, they produced a compact nanoPCR system that automatically executed reverse transcription, rapid PCR amplification, and fluorescence detection with a single button press.
The nanoPCR prototype concurrently measured three samples within 17 minutes. The limit of detection was 3.2 gene copies per microliter, which is comparable to that obtained by benchtop PCR equipment.
The investigators used the nanoPCR method to evaluate clinical samples from 75 COVID-19 patients and 75 controls). The nanoPCR device rapidly detected three gene targets (N1, N2 and RPP30) and achieved diagnostic accuracy greater than 99%. There were no false-negative or false-positive results.
Senior author Dr. Cheon Jinwoo, director of the center for nanomedicine at the Institute for Basic Science, said, "Through the improvement and miniaturization of the PCR technology, we have shown that it is possible to perform PCR based POC (point-of-care) diagnosis in the field quickly."
The nanoPCR method was described in the December 3, 2020, online edition of the journal Nature Biomedical Engineering.
Related Links:
Institute for Basic Science














