COVID-19 Fuelling Growth of In-Vitro Diagnostics Market and Creating Novel Opportunities for Players
By LabMedica International staff writers
Posted on 21 Aug 2020
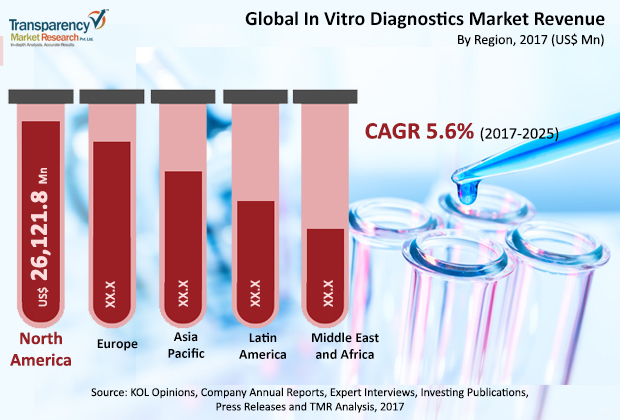
The global in-vitro diagnostics market is projected to register a compound annual growth rate of 5.60% from USD 55 billion in 2016 to nearly USD 90 billion by 2025, fueled by the massive rise in COVID -19 infections which is driving an increase in research and development activities in the diagnosis of infectious diseases. Posted on 21 Aug 2020
These are the latest findings of Transparency Market Research (Albany, NY, USA), a market intelligence provider.
Illustration
A slew of factors such as positive transformations in the medical and healthcare industries are expected to further support the market growth. Digital transformation, technological advancements and increasing significance of point-of-care diagnostics are contributing to the growth of the global in-vitro diagnostics market. State-of-the-art diagnostic facilities are enabling market growth by paving the way for better outcomes. More and more facilities are exploring advanced technologies for up-gradation purposes, thereby driving the demand for in-vitro diagnostics. Additionally, a notable increase in the incidence of cancer in several regions across the world will also contribute to the growth of the global in-vitro diagnostics market.
Furthermore, the rising incidence of infectious diseases, such as COVID-19, will act as a key driver for the growth of the global in-vitro diagnostics market. There has been an increase in the number of COVID-19 cases which is driving the demand for in-vitro diagnostic tests. The outbreak of the COVID-19 pandemic has led to investments in new research lines for diagnostic and treatment of infectious diseases. The medical industry has witnessed some success in developing diagnostic technologies for COVID-19, but still has a long way to go. As new research lines emerge for the management of the COVID-19 pandemic, the global in-vitro diagnostics market will continue to grow at a fast pace.
Geographically, the North American region is witnessing a massive increase in in-vitro testing which can be majorly attributed to the increase in COVID-19 cases in the US. In the Asia Pacific (APAC) region, cooperation between countries for better managing the increasing incidence of diseases is leading to the growth of the in-vitro diagnostics market. Rising cases of lung disorders and cancer is also supporting the market growth in several regions of the Asia Pacific.
Related Links:
Transparency Market Research













