DxGen Launches '2 Minutes' COVID-19 Antibody Testing System
By LabMedica International staff writers
Posted on 19 May 2020
A newly-launched '2 minutes' detection system for COVID-19 IgM / IgG antibodies offers reliable testing with several attractive features for medical professionals and has also received CE Mark.Posted on 19 May 2020
The newly-developed Epithod 616 analyzer and COVID-19 IgM/IgG test kit from DxGen Corp. (South Korea) is the fastest system that not only provides positive (+, ++, +++) or negative (-) results, but also shows the results through a relative numerical value in COI (Cut-Off Index), thus enabling the user to diagnose the levels of IgM and IgG antibodies present and manage their quantitative results more specifically. The Epithod 616 diagnostic system ensures better accuracy and improved sensitivity in analyzing results and eliminates the risk of undesirable misreading which frequently occurs in conventional ‘lateral-flow’ immunoassay tests where the user determines the test results through their own naked eye.
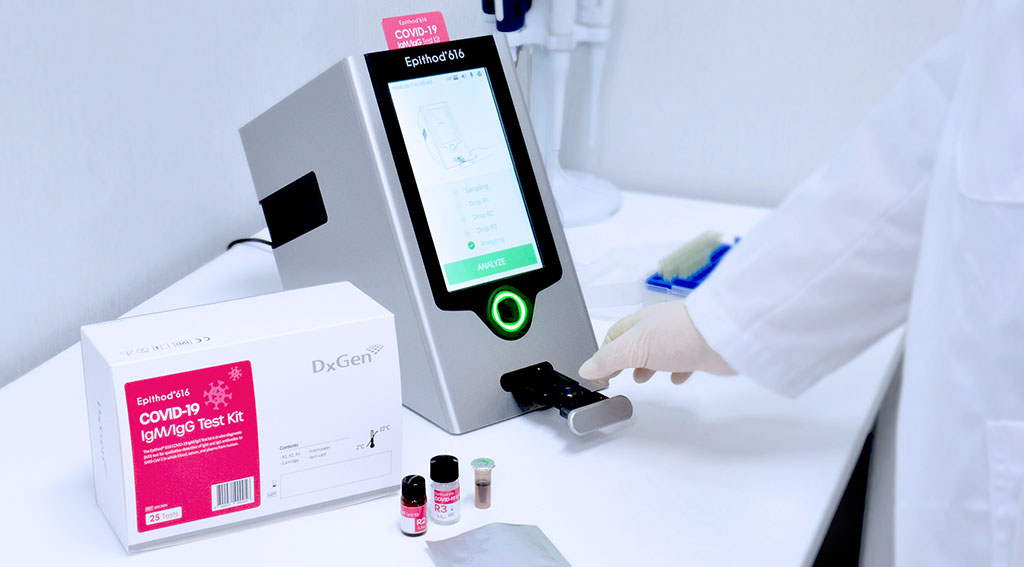
Image: The newly-developed Epithod 616 analyzer and COVID-19 IgM/IgG test kit from DxGen (Photo courtesy of DxGen Corp.)
Owing to its unique multi-chromatic reflectometry analyzer and flow-through immunoassay platform, the system can provide test results within two minutes, or more than 100 test results per hour, which significantly improves the total test capacity as compared to the conventional ‘Lateral-flow’ test kit that usually takes at least 10 minutes for each test. Additionally, the Epithod 616 is equipped with user-friendly functions, such as a ‘check-up list’ that enables the user to input patient information in detail, including fever, cough and the number of days since the onset of symptoms.
“It is very important to comprehensively monitor underlying diseases such as diabetes, myocardial infarction and pneumonia together with the COVID-19 disease in order to care the infected patients and to restrain the spread of the outbreak," said Jinwoo Lee, Ph.D., chief executive officer, DxGen Corporation. "Epithod 616 drew great attention from many potential customers who have interest in integrative diagnostics of COVID-19 with HbA1c, Glycated Albumin, CRP, hs-CRP, D-dimer and more. This is very important point to make decisions about the treatment efficacy and convalescence patients."














