Hardy Diagnostics Releases Autobio’s One-Step Test for COVID-19
By LabMedica International staff writers
Posted on 31 Mar 2020
Hardy Diagnostics (Santa Maria, CA, USA), an FDA-licensed manufacturer of medical devices for microbiological testing, has begun accepting orders for a new rapid, one-step lateral flowassay that detects both IgG and IgM antibodies to the SARS-CoV-2 virus. The immunoassay intended specifically for determining the possibility of a COVID-19 infection was developed by Autobio Diagnostics Co., Ltd. (Zhengzhou, Henan, China), a microbiology medical device manufacturer. Hardy Diagnostics has entered into a strategic partnership with Autobio Diagnostics to become a US supplier of the new in vitro diagnostic medical device, Anti-SARS-CoV-2 Rapid Test. Posted on 31 Mar 2020
The Anti-SARS-CoV-2 Rapid Test is a rapid, one-step lateral flow assay intended for the presumptive qualitative detection of IgM and IgG antibodies to the SARS-CoV-2 virus in patients suspected of a COVID-19 infection. By using a patient’s finger prick blood, serum, or plasma specimen, the Anti-SARS-CoV-2 Rapid Test offers a turnaround time of only 15 minutes. The simple-to-use test requires no equipment or special expertise or training to implement.
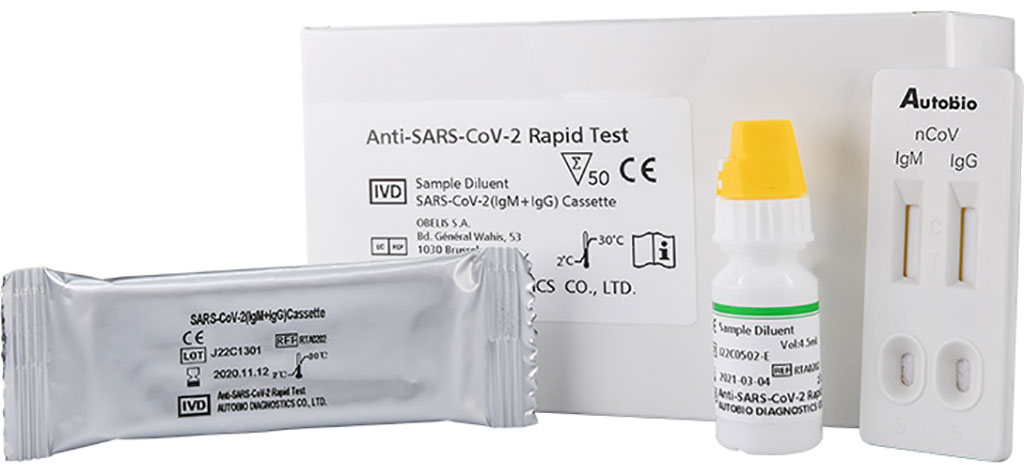
Image: Autobio’s One-Step Test for COVID-19 (Photo courtesy of Hardy Diagnostics)
Through their partnership, Hardy Diagnostics and Autobio Diagnostics have begun opening up supply chains to deliver this rapid test to the US. The rapid market deployment of the new in vitro diagnostic medical device was made possible through the FDA Emergency Use Authorization (EUA) program, allowing for its simultaneous commercialization while the product is still under review, which enables Hardy Diagnostics to supply the test during this critical time.
“We are incredibly proud of the work our partners in China have accomplished,” said Andre Hsiung, Director of Technical Services at Hardy Diagnostics. “Because Autobio quickly developed this technology and because the FDA allowed Emergency Use Authorization, we will be able to more effectively leverage our sales network to get this product out to where it is needed the most.”
Related Links:
Hardy Diagnostics
Autobio Diagnostics Co., Ltd.









 (3) (1).png)




