Chembio and LumiraDx Enter into COVID-19 Strategic Partnership
By LabMedica International staff writers
Posted on 30 Mar 2020
Chembio Diagnostics, Inc. (Medford, NY, USA), a point-of-care diagnostic company focused on infectious diseases, has entered into a worldwide strategic partnership with LumiraDx Limited (London, UK) to develop point-of-care diagnostic tests for the detection of the COVID-19 virus and IgM and IgG antibodies on both the LumiraDx and Chembio DPP platforms. Posted on 30 Mar 2020
Chembio’s patented DPP technology platform, which uses a small drop of blood from the fingertip, provides high-quality, cost-effective results in approximately 15 minutes. Coupled with Chembio’s extensive scientific expertise, its novel DPP technology offers broad market applications beyond infectious disease, a number of which applications are under active development with collaboration partners. LumiraDx designs smarter connected diagnostics and diagnostic-led care solutions to enable more effective and cost-efficient patient care. The LumiraDx Platform is an innovative, next generation point of care diagnostic system that combines a small, portable instrument, advanced low cost test strip and seamless digital connectivity.
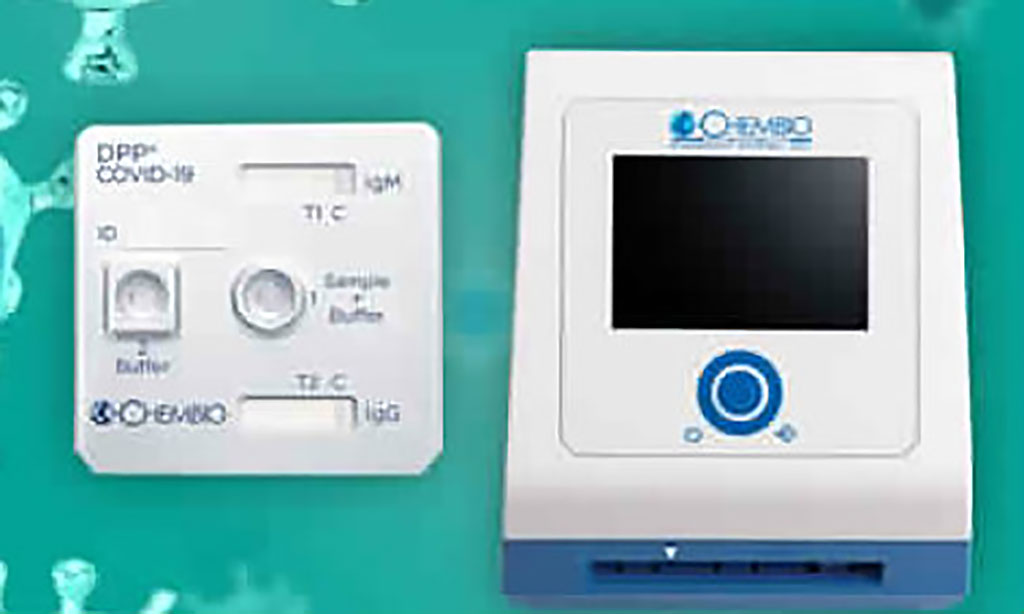
Image: The Chembio DPP platform (Photo courtesy of Chembio Diagnostics, Inc.)
“We are pleased to expand our relationship with Chembio as our partner given the company’s expertise and speed in developing high-quality point-of-care assays. By joining forces and bringing together the best of these two companies, we believe we will become the chosen approach for the detection and monitoring of the COVID-19 virus, which has become a worldwide pandemic,” said Ron Zwanziger, LumiraDx's Chairman and Chief Executive Officer.
"We are very excited to join with LumiraDx in this strategic partnership, which demonstrates our scientific expertise and the versatility of our DPP platform,” said Gail Page, Chembio's Interim Chief Executive Officer. “Through our joint efforts, we expect the new products to provide comprehensive solutions to the new demands surrounding the worldwide testing needs for COVID‑19.”
Related Links:
Chembio Diagnostics, Inc.
LumiraDx Limited














