Beryllium Causes Deadly Lung Disease
By LabMedica International staff writers
Posted on 17 Jul 2014
How the metal beryllium triggers a deadly immune response in the lungs has been discovered using detailed maps of molecular shapes and the electrical charges surrounding them. Posted on 17 Jul 2014
A genetic susceptibility to the disease creates a molecular pocket in an immune system protein, which captures beryllium ions and triggers an inflammatory response in the lungs and this immune response lies somewhere between classic forms of allergic hypersensitivity and autoimmunity.
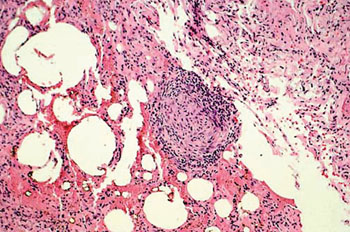
Image: Histology of lung tissue from a chronic beryllium disease patient showing granulomatous tissue (Photo courtesy of the Cleveland Clinic).
Scientists at the Howard Hughes Medical Institute (Denver, CO, USA) performed a series of highly detailed genetic, X-ray diffraction, molecular binding and electrostatic studies to show how this single amino acid can combine with other amino acids from the human leukocyte antigen (HLA)-DP2 subunit and some of its bound self-peptides to create a unique molecular pocket that captures a single beryllium ion along with a sodium ion.
The peptides that bind to HLA-DP2 come from the body's own tissues and normally elicit no immune response. With the beryllium and sodium firmly lodged in the molecular pocket, however, those peptides have a very slightly altered shape and electrical charge, which roving T cells recognize as foreign and dangerous. They initiate an immune response that causes inflammation and scarring in the lungs. The thermal stabilities of various major histocompatibility complex (MCH) alleles, the MHCII-peptide complexes, were determined by differential scanning fluorimetry using either a Stratagene MX3005p (Agilent; Santa Clara, CA, USA) or CFX96 real time polymerase chain reaction instrument (RCT-PCR, Bio-Rad; Hercules, CA, USA).
About 85% of people who develop chronic beryllium disease have the immune system protein HLA-DP2. Cells throughout the body use this molecule to tell the immune system what is going on inside of them. HLA-DP2 sits on the cell surface holding small protein fragments taken from the cell's interior. Immune system sentinels known as T cells collide against HLA-DP2 and its displayed protein fragment. If the protein fragment is derived from the body's own proteins, the T cell ignores it; if it is a foreign peptide, say from a bacterium, virus or other pathogen, the T cell sounds the alarm and triggers an immune response.
John W. Kappler, PhD, a professor of immunology and the senior author of the study said, “This response resembles allergic hypersensitivity in that a metal ion causes an allergic reaction. But it also resembles autoimmunity in that the immune system is mounting an attack against a self-peptide. It is a new form of immune response, and may lead to new therapeutic strategies to treat and prevent the disease.” The study was published on July 3, 2014, in the journal Cell.
Related Links:
Howard Hughes Medical Institute
Agilent
Bio-Rad













