Saliva-Based Cancer Detection Technology Eliminates Need for Complex Sample Preparation
Posted on 24 Jul 2025
Early detection of cancer and other serious diseases is crucial for effective treatment and improved outcomes, yet current diagnostic methods often involve invasive procedures and complex sample preparation. These barriers limit accessibility and delay diagnosis, particularly in low-resource settings. For example, detecting tumor-specific mutations typically requires blood samples or tissue biopsies, which are time-consuming and costly. A newly developed saliva-based platform offers a promising alternative by allowing non-invasive detection of disease biomarkers with high accuracy. In earlier studies, the technology demonstrated strong sensitivity and specificity in identifying tumor mutations, particularly for non-small cell lung cancer, directly from saliva.
Researchers at the UCLA School of Dentistry (Los Angeles, CA, USA) developed a diagnostic platform known as EFIRM (Electric Field-Induced Release and Measurement), a liquid biopsy technology designed to isolate and analyze biomarker signals from biofluids such as saliva. The platform, which builds on more than two decades of research, eliminates the need for complex preparation and uses proprietary electric field-induced techniques to detect disease-related genetic material. The development of EFIRM has led to clinical advances in the detection of salivary biomarkers and has positioned the technology as a potential game changer in early, non-invasive diagnostics. The UCLA School of Dentistry has now entered a three-year sponsored research agreement with Dongwoon Anatech (Seoul, South Korea) to further optimize and clinically validate EFIRM for broader medical applications.
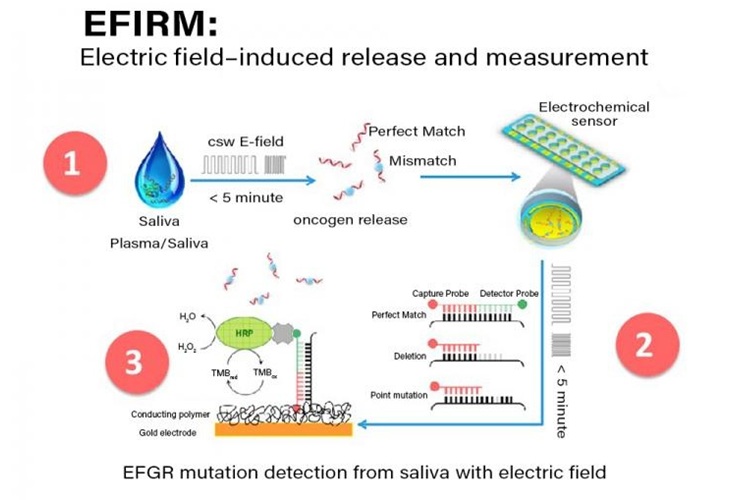
The collaboration will begin by refining protocols for D-SaLife, Dongwoon’s saliva-based glucose monitoring system, in diabetic and non-diabetic patients using samples from hospitals in the U.S. and South Korea. Later phases will focus on developing EFIRM I, a fully automated diagnostic device for cancers such as lung, gastric, and oral cancer, and co-developing EFIRM II, a next-generation, semiconductor-based platform with compact diagnostic cartridges for scalable, point-of-care use. While earlier studies confirmed the system’s clinical accuracy, the current research partnership will allow for expanded testing and device development aimed at real-world deployment. Researchers plan to evaluate performance across different populations, integrate semiconductor technologies for enhanced sensitivity, and eventually bring the system to clinical practice at scale.
"This collaboration reflects our shared vision to make early disease detection faster, simpler, and more accessible around the world," said Dr. David T.W. Wong, professor at the UCLA School of Dentistry. “Dongwoon Anatech’s support brings us closer to realizing EFIRM’s potential in clinical settings.”
Related Links:
UCLA School of Dentistry
Dongwoon Anatech














