Impact of Preanalytical Factors on Calprotectin Concentration in Stool
Posted on 29 Dec 2022
In inflammatory bowel disease (IBD), fecal calprotectin measurement is increasingly important in selecting patients for diagnostic endoscopy, monitoring of disease activity, and evaluation of treatment response.
Calprotectin is a calcium-binding protein mainly produced in neutrophils. It is said to be resistant to bacterial degradation in the colon, and the literature almost unanimously states that calprotectin is stable up to seven days at room temperature without preservation buffer.
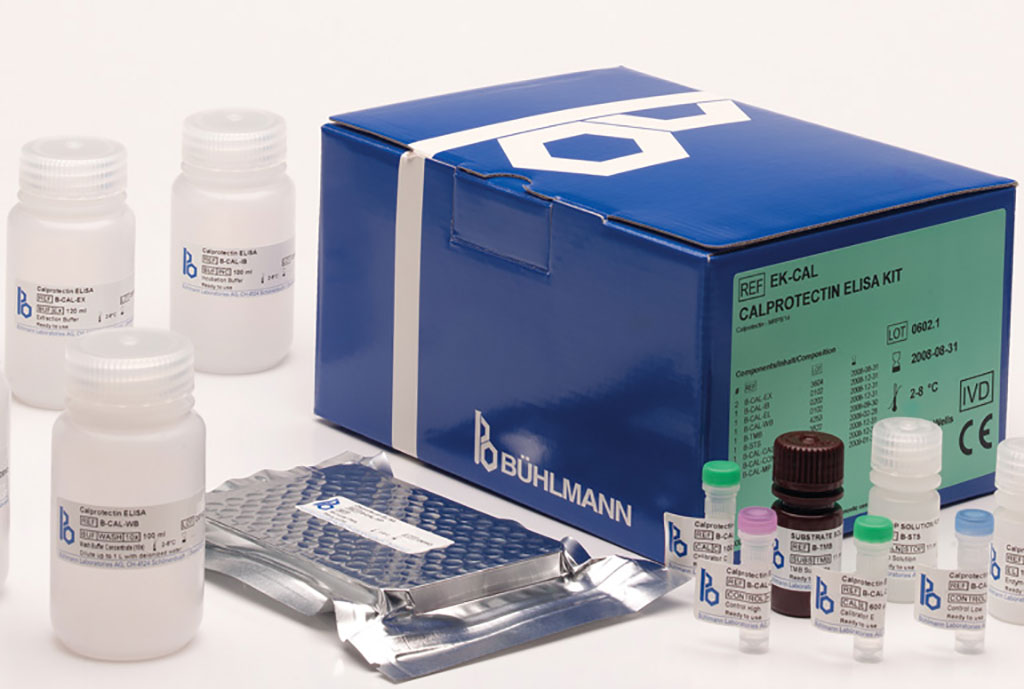
Clinical Chemists at the University Medical Center Groningen (Groningen, The Netherlands) and their colleagues from other institutions evaluated the impact of pre-analytical storage conditions on reliability of calprotectin testing using five different calprotectin immunoassays. The scientists distributed 45 frozen anonymized feces aliquots among the three participating centers. They assessed the calprotectin concentration over time under four conditions, including (a) untreated native stool stored at room temperature (NRT), (b), stool extract stored at room temperature, (c), untreated native stool stored at 4 °C, and (d), stool extract stored at 4 °C.
The five assays were: the Bühlmann fCAL turbo test (Bühlmann Laboratories AG, Schönenbuch Switzerland) is a particle-enhanced turbidimetric immunoassay performed on a COBAS 6000 e501 (Roche Diagnostics, Rotkreuz. Switzerland); the Bühlmann fCAL enzyme-linked immunosorbent assay (ELISA; Bühlmann Laboratories AG) is a sandwich-based ELISA performed and analyzed using a DS2 Dynex ELISA robot (Dynex, Chantilly, VA, USA); the CALliaGold test (Sentinel CH, Milan Italy) is a particle-enhanced turbidimetric immunoassay (PETIA) and analysis was performed using a SENTiFIT 270 Analyzer (Sysmex Europe SE, Norderstedt, Germany).
The other two assays were the EliA Calprotectin test which is a fluorescence enzyme immunoassay and the analysis was performed using the Phadia 250 (Thermo Fisher Scientific, Waltham, MA, USA); and the QUANTA Flash Calprotectin (Inova Diagnostics , San Diego, CA, USA) a chemiluminescent immunoassay and the analysis was performed on BIO FLASH (Werfen, Bedford, MA, USA).
The investigators reported that Calprotectin concentrations declined over time under all pre-analytical conditions with all assays, except for extracted feces stored at 4 °C. The rate of decline was greatest in untreated stool kept at room temperature, reaching significant difference from baseline already after one day. In extracted feces kept at room temperature, significant difference from baseline was reached after two days, and in untreated feces at 4 °C, after four days. However, the results differed significantly between assays. After four days of storage at room temperature, the mean calprotectin decline from baseline differed between 30% and 60%, dependent on the assay used. In most, but not for all samples, the CALiaGold assay produced the highest calprotectin levels, and the QUANTA flash assay, the lowest calprotectin levels.
The authors conclude that fecal calprotectin concentration in stool samples declines over time, and the rate of decline is greater at higher temperatures. In extracted feces stored at 4 °C, calprotectin is the most stable. It is assay-dependent how long extracted feces stored at 4 °C give reliable test results. The study was published in the November 2022 issue of The Journal of Applied Laboratory Medicine.
Related Links:
University Medical Center Groningen
Bühlmann Laboratories
Roche Diagnostics
Dynex
Sentinel CH
Sysmex
Thermo Fisher Scientific
Inova Diagnostics
Werfen