Blood Test Aids Diagnosis of Appendicitis
By LabMedica International staff writers
Posted on 22 Jan 2015
Clinical trial results have been announced for a rapid blood test for aiding in identifying children, adolescent, and young adult patients in the emergency room who are at low probability for appendicitis.Posted on 22 Jan 2015
The unique appendicitis test has projected high sensitivity and negative predictive value and is being developed to aid in the identification of patients at low probability for acute appendicitis, allowing for more conservative patient management.
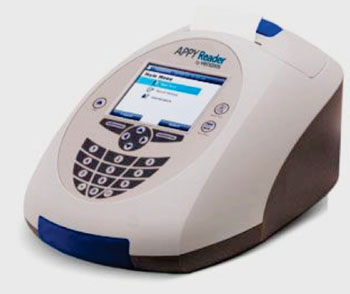
Image: The APPYReader, a compact, automated fluorescent immunoassay instrument for calculating APPY 1 test results (Photo courtesy of Venaxis).
The trial involved 29 hospital sites in the USA and recruited patients aged 2 to 20 from January 2013 through January 2014 and included 1,887 patients with suspected acute appendicitis. The APPY1 Test (Venaxis, Inc.; Castle Rock, CO, USA) measures the concentrations of myeloid-related protein MRP 8/14 (calprotectin) and C-reactive protein (CRP) in EDTA-plasma by lateral flow immunoassay. MRP 8/14, CRP, and a manually entered WBC count are then computed by the reader’s preprogrammed proprietary algorithm to give an APPY1 Test result and a qualitative interpretation to facilitate the utility of the results. The APPYReader measures and generates the results of the APPY1 Test. The reader is a compact, automated fluorescent immunoassay instrument that calculates individual protein biomarker concentrations of MRP 8/14 and C-reactive protein (CRP).
The results of the study showed that the APPY1 Test exhibited a sensitivity of 96.9% (95% CI, 94.9%–98.1%), a negative predictive value of 97.3% (95% CI, 95.5%–98.3%), a negative likelihood ratio of 0.08 (95% CI, 0.05%–0.14), and a specificity of 37.8% (95% CI, 35.5%–40.4%) for acute appendicitis. The prevalence of the disease was 25.3%. The panel correctly identified 533 of 1,409 (37.8%) patients who did not have appendicitis with 15 (3.1%) false negatives among 478 patients with acute appendicitis. Among patients without appendicitis, 32% (136/431) who had X-ray computed tomography (CT) scans were correctly identified by negative APPY1 Test results.
Steve Lundy, President and CEO of Venaxis, said, “The increased risk of radiation induced cancers associated with CT scans is of particular concern in younger patients, due to their size, radiosensitivity, and longer spans of time to develop these cancers. We believe the results of the trial reveal our test to be of important future value as a means to reduce radiation exposure in children.” The study was presented at the American College of Emergency Physicians Scientific Assembly 2014 (ACEP14) held October 27 to October 30, 2014, in Chicago (IL, USA).
Related Links:
Venaxis, Inc.