Chembio Launches DPP COVID-19 Serological Point-of-Care Test
By LabMedica International staff writers
Posted on 03 Apr 2020
Chembio Diagnostic Systems, Inc. (Medford, NY, USA), a point-of-care diagnostic company focused on infectious diseases, has launched its rapid DPP COVID-19 serological point-of-care test for the detection of IgM and IgG antibodies in the US. The results can be obtained within 15 minutes from a simple finger stick utilizing Chembio’s MicroReader 1 and MicroReader 2 analyzers. The ability of the DPP platform to provide numerical results can aid clinicians in determining current or past exposure to the COVID-19 virus and monitoring infection progression, while avoiding the human interpretation errors associated with visual readings. Posted on 03 Apr 2020
The company’s patented DPP technology platform, which uses a small drop of blood from the fingertip, provides high-quality, cost-effective results in approximately 15 minutes. Coupled with Chembio’s extensive scientific expertise, its novel DPP technology offers broad market applications beyond infectious disease, a number of which applications are under active development with collaboration partners.
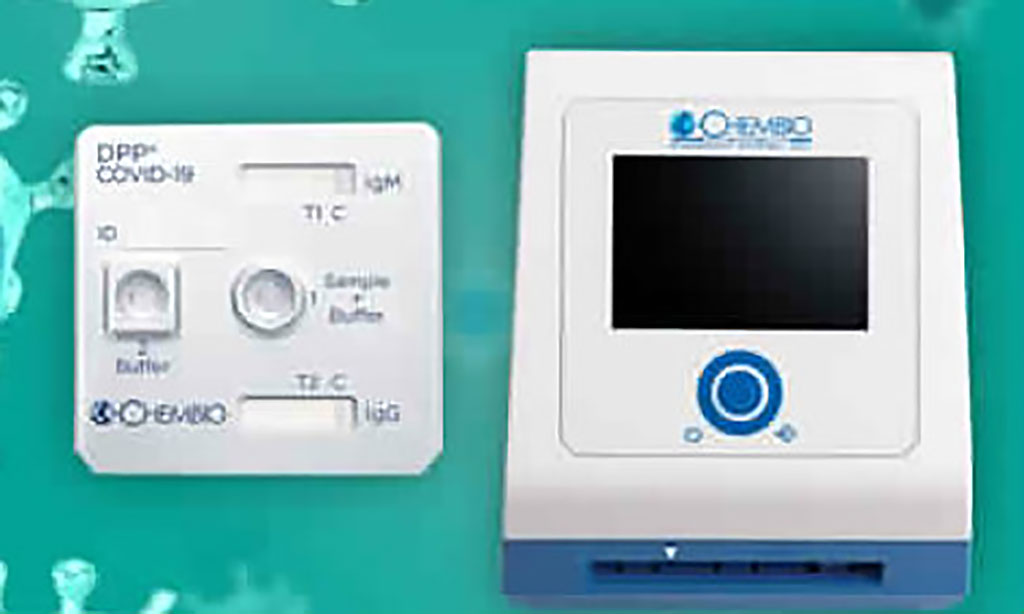
Image: The Chembio DPP platform (Photo courtesy of Chembio Diagnostics, Inc.)
Chembio’s DPP COVID-19 test detects antibodies in the blood that are produced by the body in response to a novel coronavirus infection. Chembio has also entered into a worldwide strategic partnership with LumiraDx Limited (London, UK) to develop point-of-care diagnostic tests for the detection of the COVID-19 virus and IgM and IgG antibodies on both the LumiraDx and Chembio DPP platforms.
“The results and data from our DPP COVID-19 test can help improve clinical outcomes through the management of individual patients by enabling clinicians to understand the likelihood of past and present infection and to manage populations as a whole as a surveillance test,” said Richard Eberly, Chief Executive Officer of Chembio. “Our measured approach has positioned us to offer a viable and sustainable long-term solution for clinicians. We expect to begin shipping product in April 2020, and we will continue to work with our partner LumiraDx to provide DPP COVID-19 tests with the ability to scale based upon market demand.”
Related Links:
Chembio Diagnostic Systems, Inc.
LumiraDx Limited












