Blood Test to Help Low-Risk Gastric Cancer Patients Avoid Unnecessary Surgery
Posted on 09 Dec 2025
Accurately identifying lymph node metastasis in early-stage gastric cancer remains a major clinical challenge. CT imaging often misses up to half of lymph node–positive cases, leading clinicians to recommend radical gastrectomy as a precaution despite low actual metastasis rates. Many patients undergo unnecessary invasive surgery, even though most early-stage cases qualify for endoscopic treatment with excellent survival outcomes. A new liquid biopsy model now predicts metastasis risk more reliably to support safer treatment selection.
Researchers at the Institute of Science Tokyo (Tokyo, Japan) have created a blood-based assay using DNA methylation biomarkers combined with CT imaging data. The team conducted genome-wide analyses to identify six methylation markers linked to metastatic behavior in early gastric cancer. These markers were then integrated with preoperative CT findings to train a predictive model capable of differentiating high-risk from low-risk cases.
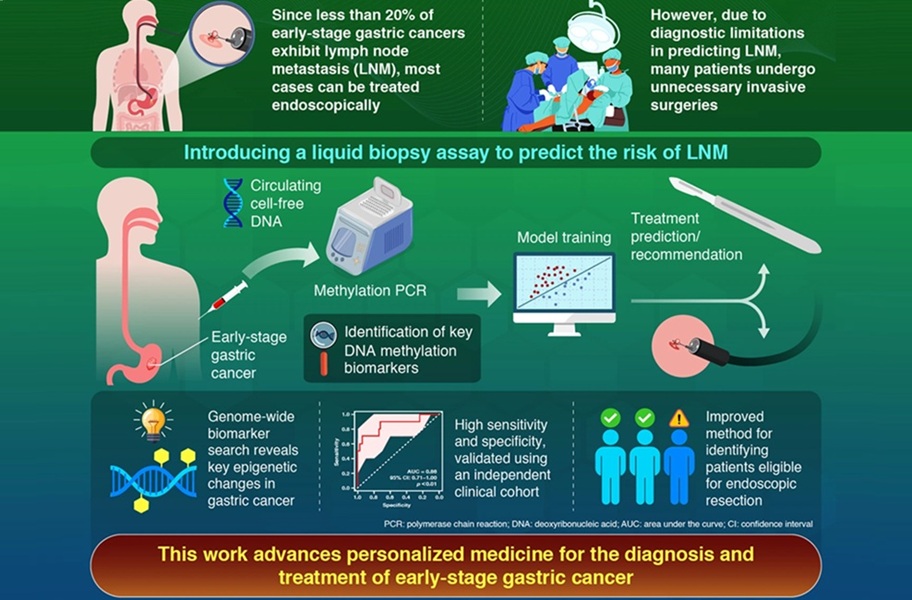
The method identifies epigenetic alterations in plasma that reflect early metastasis-related changes, offering a dynamic view of tumor biology not accessible through imaging alone. By incorporating both molecular and structural information, the model enhances diagnostic accuracy while remaining noninvasive. In retrospective testing, the model demonstrated high specificity and strong predictive ability, accurately distinguishing patients who did not require radical surgery.
The results, published in the United European Gastroenterology Journal, showed that treatment decisions could be improved significantly, reducing unnecessary surgical procedures without missing any true metastasis cases. This indicates strong potential for the model to alter standard preoperative evaluation and reduce overtreatment. Looking ahead, large-scale multicenter trials are planned to validate performance, support regulatory pathways, and establish the test as a routine diagnostic option for early gastric cancer.
“In contrast to the current clinical standard, our model allowed about 44% of patients to avoid unnecessary surgery without missing any LNM-positive patients,” said Assistant Professor Keisuke Okuno, lead developer of the study. “We expect our model to advance personalized medicine for early-stage GC by improving the accuracy of treatment selection.”
Related Links:
Institute of Science Tokyo









 Analyzer.jpg)



