Novel Gene Therapy Based on Modified Version of CRISPR/Cas9 Tool
By LabMedica International staff writers
Posted on 21 Dec 2017
A modified form of the CRISPR/Cas9 gene-editing tool does not cause "double-strand breaks" (DSBs) in the DNA, yet it retains the ability to target and activate specific sites in the genome.Posted on 21 Dec 2017
CRISPR/Cas9 is regarded as the cutting edge of molecular biology technology. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. CRISPRs are found in approximately 40% of sequenced bacteria genomes and 90% of sequenced archaea. CRISPRs are often associated with cas genes that code for proteins related to CRISPRs.
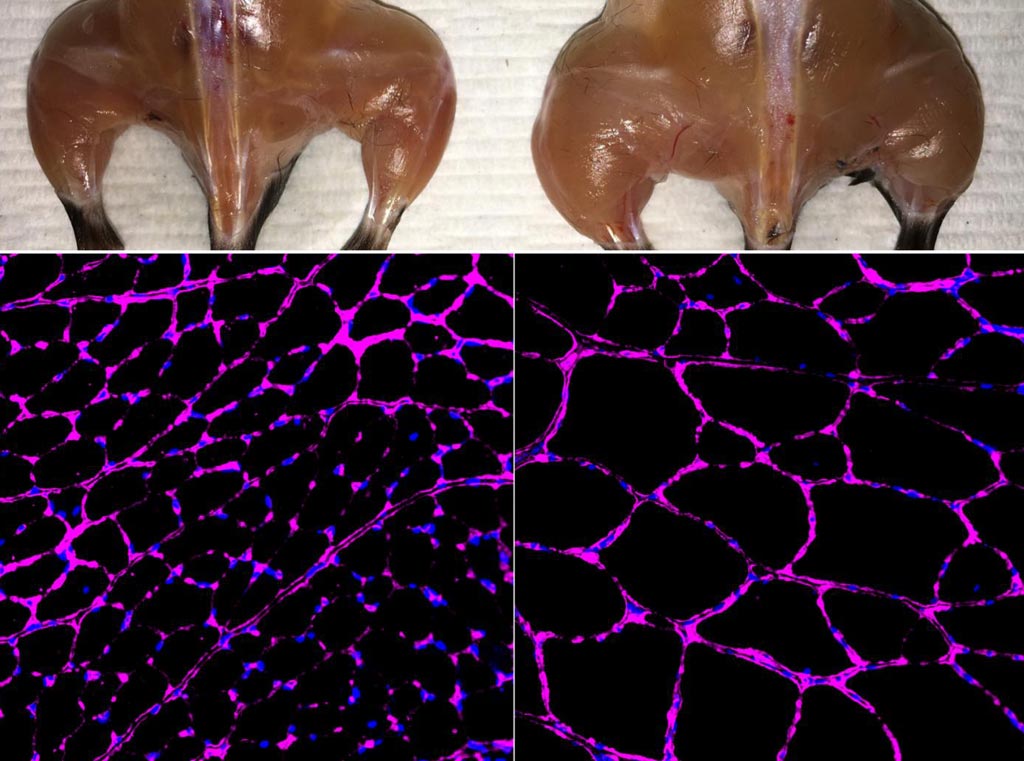
Image: An advanced in vivo Cas9-based epigenetic gene activation system enhances skeletal muscle mass (top) and fiber size growth (bottom) in a treated mouse (right) compared with an independent control (left). The fluorescent microscopy images at bottom show purple staining of the laminin glycoprotein in tibialis anterior muscle fibers (Photo courtesy of the Salk Institute for Biological Research).
Since 2013, the CRISPR/Cas system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (gRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand.
In a paper published in the December 7, 2017, online edition of the journal Cell, investigators at the Salk Institute for Biological Research (La Jolla, CA, USA) used a modified version of CRISPR/Cas9. This version is based on a catalytically inactive form of Cas9 (dCas9), which can still target specific sites in the genome, but no longer induces DSBs that cut DNA. CRISPR/dCas9 associates with transcriptional activation domains, which are molecular switches that activate targeted genes.
Delivery of the CRISPR/dCas9 complex required development of a new methodology, as it was too large to fit into the commonly used adeno-associated virus (AAV) transport system. Therefore, the investigators separated the editing tool into its two primary components by generating a dual-AAV system based on co-injection of AAV-dCas9 with a separate AAV-gRNA.
The investigators used this technique to treat mouse models of diabetes, muscular dystrophy, and acute kidney disease. Results demonstrated that CRISPR/dCas9-mediated target gene activation could be achieved in vivo, leading to measurable phenotypes and amelioration of disease symptoms.
"Although many studies have demonstrated that CRISPR/Cas9 can be applied as a powerful tool for gene therapy, there are growing concerns regarding unwanted mutations generated by the double-strand breaks through this technology," said senior author Dr. Juan Carlos Izpisua Belmonte, a professor in the gene expression laboratory at the Salk Institute for Biological Research. "We were able to get around that concern."
Related Links:
Salk Institute for Biological Research














