Combined Gene and Immunotherapy Shows Potential for Aggressive Brain Tumors
By LabMedica International staff writers
Posted on 17 Jan 2017
A novel approach that combined gene and immunotherapy demonstrated considerable potential in a mouse model as a method for treating the aggressive brain tumor glioblastoma multiforme (GBM).Posted on 17 Jan 2017
GBM is the most common primary tumor of the central nervous system and is almost always fatal. The aggressive invasion of GBM cells into the surrounding normal brain makes complete surgical removal impossible, significantly increases resistance to the standard therapy regimen, and virtually assures tumor recurrence. Median survival for newly diagnosed GBM is 14.6 months and declines to eight months for patients with recurrent GBM.
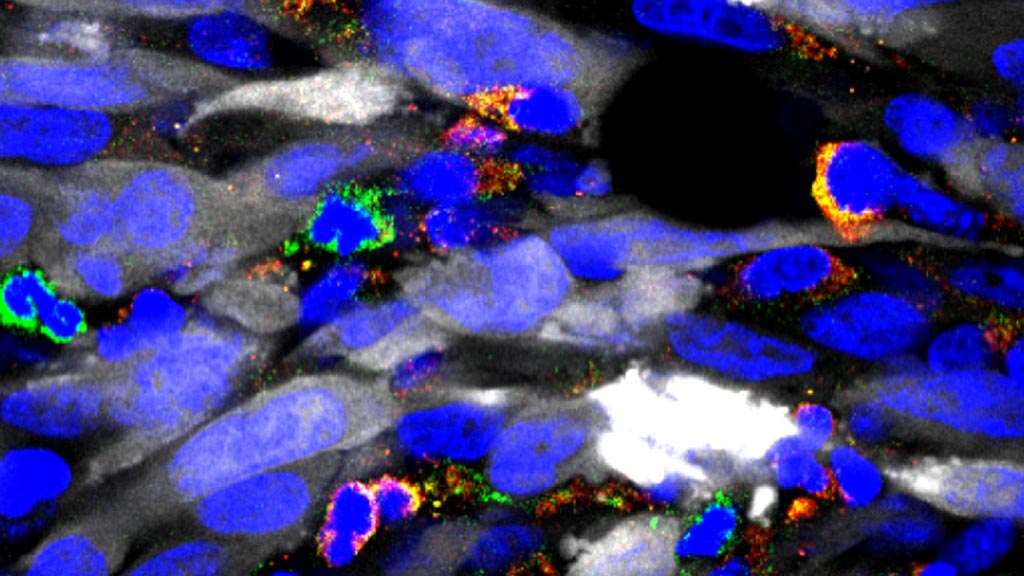
Image: A photomicrograph showing numerous immune suppressive cells in green and yellow, infiltrating the tumor mass (tumor cells in white). By depleting these immune suppressive cells, immunotherapy becomes much more effective (Photo courtesy of the University of Michigan).
While immunotherapeutic approaches that harness the cytotoxic and memory potential of the host immune system have shown great benefit in other types of cancer. GBMs have developed multiple strategies, including the accumulation of myeloid-derived suppressor cells (MDSCs) to induce immunosuppression. MDSCs contribute to an immunosuppressive network that protects tumors by disabling T-cell adaptive immunity.
As it is imperative to develop multipronged approaches when aiming to generate a robust anti-tumor immune response, end investigators at the University of Michigan tested whether combining MDSC depletion or checkpoint blockade would increase the efficacy of immune-stimulatory herpes simplex type-I thymidine kinase (TK) plus Fms-like tyrosine kinase ligand (Flt3L)-mediated immune stimulatory gene therapy. The method used by the investigators on a mouse GBM model required injecting adenovirus vectors carrying herpes simplex 1 thymidine kinase into the tumor, followed by an antiviral, to elicit tumor cell death. This treatment was used in combination with another adenovirus vector carrying an immune stimulatory cytokine to recruit immune cells into the tumor.
Results published in the January 4, 2017, online edition of the journal Molecular Therapy revealed that MDSCs constituted more than 40% of the tumor-infiltrating immune cells. These cells expressed IL-4Ralpha, inducible nitric oxide synthase (iNOS), arginase, programmed death ligand 1 (PDL1), and CD80, molecules that are critically involved in antigen-specific T-cell suppression.
Depletion of MDSCs strongly enhanced the TK/Flt3L gene therapy-induced tumor-specific CD8 T-cell response, which led to an increased median survival and percentage of long-term survivors. Also, combining PDL1 or CTLA-4 immune checkpoint blockade greatly improved the efficacy of TK/Flt3L gene therapy.
"For the first time, we proved that a type of immunosuppressive cells within the tumor environment play a major role in determining the impact of immunotherapies," said senior author Dr. Maria Castro, professor of neurosurgery and cell and developmental biology at the University of Michigan. "We hope the implementation of our gene therapy strategy for gliomas, used in combination with immune checkpoint blockade, will eventually provide successful treatment for patients with this devastating brain cancer."













