LumiraDx Introduces Its Innovative and Disruptive LumiraDx Platform at AACC Clinical Lab Expo
Posted on 27 Sep 2021
LumiraDx (London, UK) introduced the LumiraDx Platform, its innovative, disruptive solution designed to deliver lab-comparable performance to transform community care, at the 2021 AACC Annual Scientific Meeting & Clinical Lab Expo.
AACC's annual meeting which was held this year in Atlanta on September 26-30 is the world's largest lab medicine conference with nearly 20,000 participants from more than 100 countries.
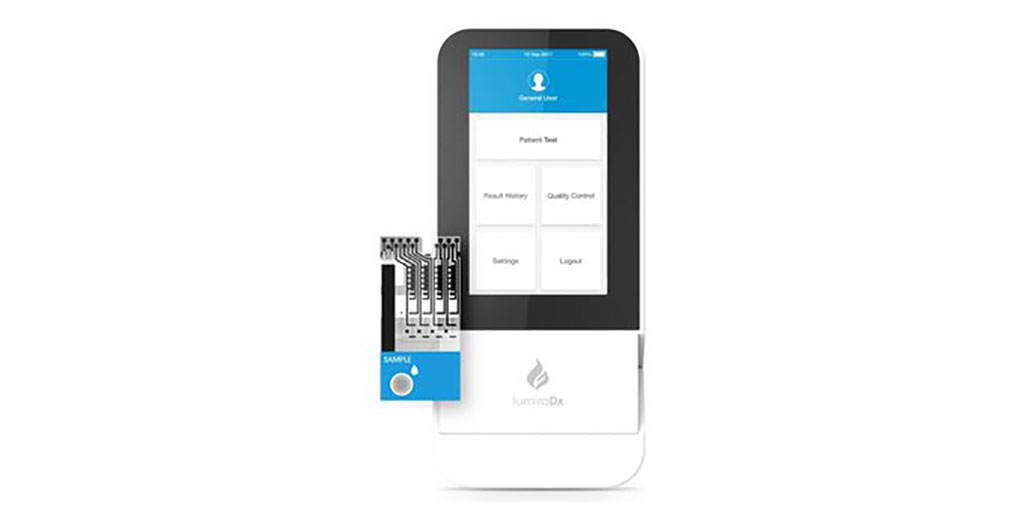
The LumiraDx Platform is an innovative, next generation point of care diagnostic system that combines a small, portable instrument, advanced low cost test strip and seamless secure digital connectivity to the Cloud and hospital IT systems. The LumiraDx Platform and tests are designed on the same principles as lab analyzer systems, to deliver accurate results compared to laboratory reference assays across a number of parameters, in a portable, easy-to-use point of care solution. Easy and intuitive to use, it requires only basic training and is designed to be affordable and accessible at the point of care
LumiraDx has developed a high sensitivity antigen test for COVID-19 on the LumiraDx Platform. The LumiraDx COVID-19 antigen test has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) and achieved CE Mark. The LumiraDx Platform and COVID-19 antigen tests are also available in Japan and Brazil and being rolled out in more than 60 countries globally. The LumiraDx Platform menu also includes point of care tests for COVID-19 Antibody, INR and D-Dimer - with high levels of accuracy comparable to central lab-based tests - all of which have achieved CE Mark and are commercially available in Europe. The Platform is designed to go wherever the patient is, whether this is in a hospital, medical office, pharmacy, or in other non-traditional settings such as schools or airports.
Related Links:
LumiraDx