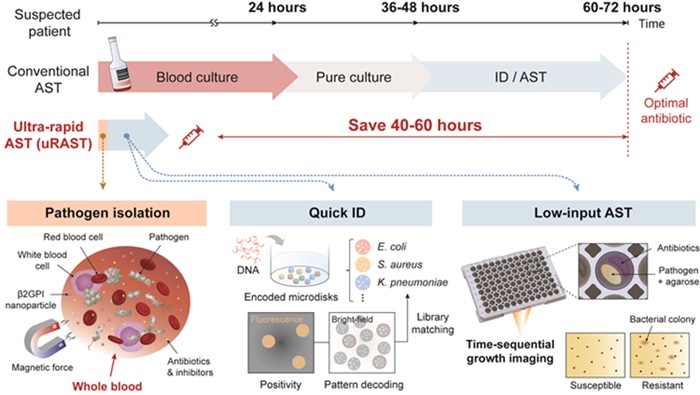
Ultra-Rapid Culture-Free Sepsis Test Reduces Testing Time from Days to Hours
An ultra-rapid antimicrobial susceptibility testing (AST) method that bypasses the need for traditional blood culture has demonstrated the potential to reduce the turnaround time of reporting drug susceptibility profiles by more than 40–60 hours compared with hospital AST workflows. More...26 Jul 2024

Ultra-Rapid Culture-Free Sepsis Test Reduces Testing Time from Days to Hours
An ultra-rapid antimicrobial susceptibility testing (AST) method that bypasses the need for traditional blood culture has demonstrated the potential to reduce the turnaround time of reporting drug susceptibility profiles by more than 40–60 hours compared with hospital AST workflows. More...26 Jul 2024
New Rapid Method for Determining Virus Infectivity Could Revolutionize Response to Future Pandemics
A new groundbreaking assay can screen viruses against virucidal antivirals in minutes, enabling quick determination of the effectiveness of antiviral measures, such as disinfectants. More...25 Jul 2024
Simple Blood Test Identifies Multiple Myeloma Patients Likely to Benefit from CAR-T Immunotherapy
A simple blood test that counts lymphocytes (a type of white blood cell) to predict the success of CAR-T immunotherapy in patients with relapsed multiple myeloma will allow physicians to pursue alternative treatments more quickly. More...25 Jul 2024

New Rapid Method for Determining Virus Infectivity Could Revolutionize Response to Future Pandemics
A new groundbreaking assay can screen viruses against virucidal antivirals in minutes, enabling quick determination of the effectiveness of antiviral measures, such as disinfectants. More...25 Jul 2024
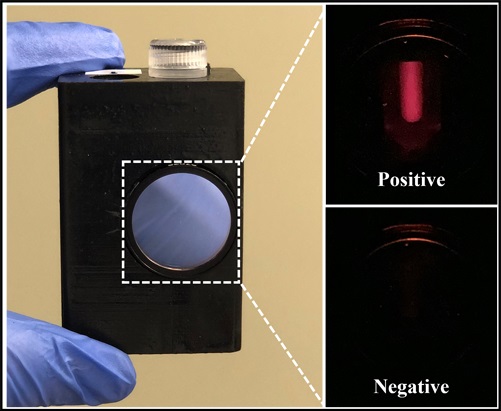
Handheld Device Puts Power of Lab-Based Diagnostic Testing in the Palm of Your Hand
Researchers have developed a platform technology that incorporates PCR-like capabilities within a handheld device, producing results within about an hour compared to the longer processes associated with traditional PCR. More...24 Jul 2024
In Other News
AI Model Identifies Breast Tumor Stages Likely To Progress to Invasive Cancer
Protein Signatures in Blood Can Predict Risk of Developing More Than 60 Diseases
Portable PCR Platform to Detect Multiple Pathogenic Bacteria Targets and Antibiotic Susceptibility at POC
Computational Tool Integrates Transcriptomic Data for Improved Breast Cancer Diagnosis and Treatment
Screening Tool Detects Multiple Health Conditions from Single Blood Drop
Screening Tool Detects Multiple Health Conditions from Single Blood Drop
Computational Tool Integrates Transcriptomic Data for Improved Breast Cancer Diagnosis and Treatment
Join TianLong at ADLM 2024 to Explore the Latest Diagnostic Innovations!
World’s First Rapid Diagnostic Test Detects Stroke within Minutes at POC
Exhaled-Breath Test Shows Promise for Detection of Lung Cancer
Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
Novel Molecular Test to Help Prevent and Control Multi Drug-Resistant Fungal Pathogen in Healthcare Settings
Novel Molecular Test to Help Prevent and Control Multi Drug-Resistant Fungal Pathogen in Healthcare Settings
Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
Early Detection of miRNAs in Maternal Blood Could Predict Preeclampsia
New Analytical Tool Could Replace Traditional Biopsies with Liquid Biopsies for Disease Diagnosis
Pocket-Sized Invention Revolutionizes Ability to Swiftly Detect Pathogens in Hospital Setting
Beckman Coulter Licenses Alzpath's Proprietary P-tau 217 Antibody to Develop Alzheimer's Blood Test
AI Identifies Drug-Resistant Typhoid-Like Infection from Microscopy Images within Hours
Innovative C. Difficile Diagnostic Test Provides Both GDH and Toxin Results within 30 Minutes
AI Identifies Drug-Resistant Typhoid-Like Infection from Microscopy Images within Hours










 Reagent.jpg)
