
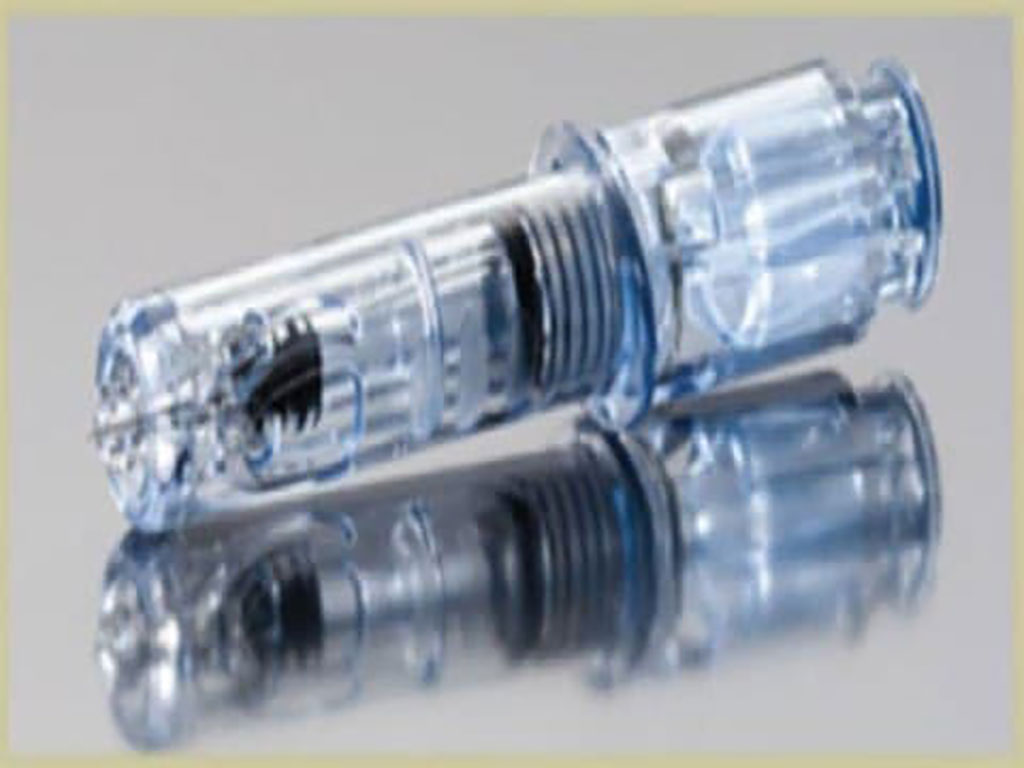

Thermo Fisher’s New Saliva Sample Collection Method for High-Throughput, Automated COVID-19 Testing System Granted FDA EUA
Thermo Fisher Scientific Inc. (Waltham, MA, USA; www.thermofisher.com) has received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) to run COVID-19 tests from saliva samples collected with the Spectrum Solutions SpectrumDNA SDNA-1000 collection device on the Amplitude Solution. More...19 Oct 2021

Cell Fitness Status Detected from Nasal Swab Test Could Accurately Predict Hospitalization or Death in COVID-19 Patients
In the future, billions of COVID-19 nasal swab tests could not only tell if you have contracted COVID-19, but maybe even foretell if you are in danger of a serious infection. More...19 Oct 2021
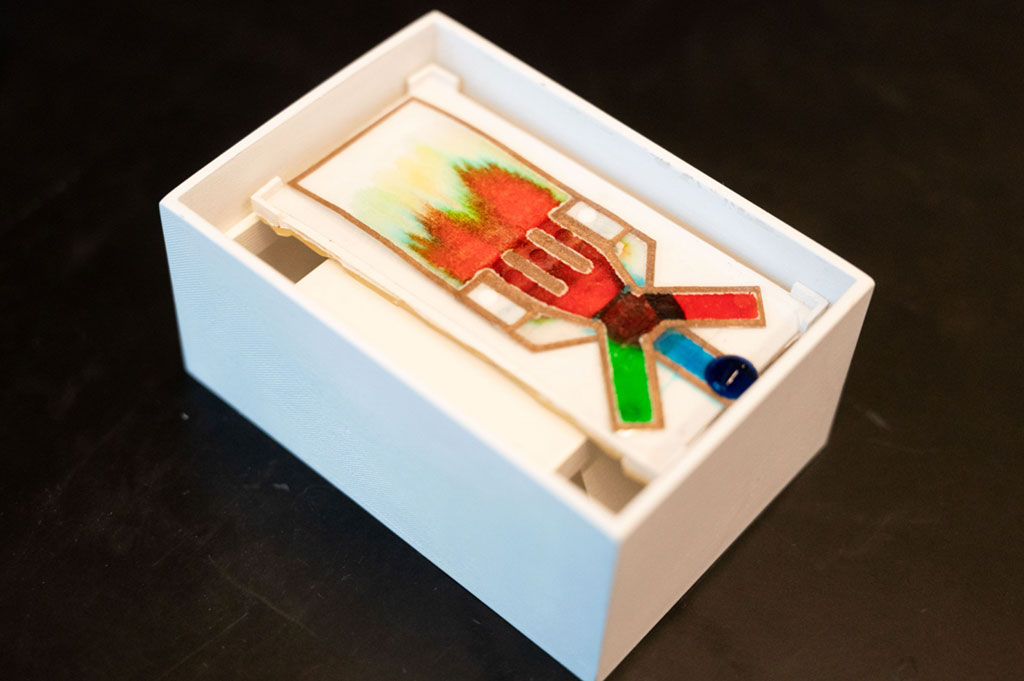

Newly-Launched Portable Molecular Diagnostic Platform Detects COVID-19 at Point-of-Care within 30 Minutes
A new portable molecular diagnostic platform and its compatible diagnostic test for the detection of SARS-CoV-2 delivers results within 30 minutes at the Point-of-Care (POC). More...15 Oct 2021
In Other News
Lateral Flow Tests for COVID-19 More Accurate than Previously Reported, Cannot Be Compared to PCR Tests
New Nanomechanical Technique for Fast, One-Step, Immune-Affinity Tests Rapidly Quantifies Transmissibility of COVID-19 Variants
Pooled Saliva Testing for COVID-19 Can Be Cheaper and as Accurate as Individual Nasopharyngeal Diagnostic Tests
Next Generation SARS-CoV-2 Test Measures Response to COVID-19 Vaccine and Neutralizing Antibody Levels in 10 Minutes
Study Examines Proteins in Saliva to Predict COVID-19 Severity Risk in Children with Help of AI Model
CT Values Should Not Be Used to Assess Performance of SARS-CoV-2 PCR Tests or Triage COVID-19 Patients, Finds Study
New SARS-CoV-2 Kit Simultaneously Detects Four COVID-19 Variants with Single RT PCR Test
Study Questions Popular COVID-19 Test and Proposes New Marker of Disease Severity
EUROIMMUN Anti-SARS-CoV-2 S1 Curve ELISA Granted FDA Emergency Use Authorization
New Stool-Based Assay Detects SARS-CoV-2 Genetic Material in Donor Stool Using RT-PCR
T-Cell Tests Unreliable in Establishing Previous COVID-19 Infection, Finds New Study
First-of-Its-Kind SARS-CoV-2 Antibody Testing System Tests Both Anti-Nucleocapsid and Anti-Spike Antibodies in One Reaction
New COVID-19 Antigen Testing Method Offers Highly Accurate Results in Less than Three Minutes
Blood Test for Combined Measurements of WBCs and Biomarkers Can Predict COVID-19 Severity
Novel Study on Performance of Coronavirus Tests in Children Presented at 2021 AACC Annual Scientific Meeting
New Rapid Test That Identifies Deteriorating COVID-19 Patients with Greater Accuracy than Existing Tests Presented at AACC 2021
New Blood Test for Occupational Stress Identifies Healthcare Professionals Burned out from COVID-19 Pandemic
Novel Insights on COVID-19 Vaccines and Virus Evolution, AI in the Clinic, and Miniaturization of Diagnostic Platforms Explored at AACC 2021
Seegene Unveils New STARlet-AIOS All-in-One Solution for All Molecular Testing at AACC 2021
NGeneBio Showcases NGS-Based Oncology/Genetic Diseases Kits and NGenePlex nCoV qRT-PCR Kit Against COVID-19
Visby Medical Presents New Portable PCR COVID-19 Test Kit at 2021 AACC Annual Scientific Meeting & Clinical Lab Expo
Fluxergy Introduces First-Of-Its-Kind Multi-Modal Laboratory Platform That Diagnoses COVID-19 On-Site in 60 Minutes
Mammoth Biosciences Presents AACC 2021 Disruptive Technology Award Finalist CRISPR-Based Detection Platform











