Researchers Discover World’s First Human Antibody that Could Inhibit New Coronavirus (SARS-CoV-2)
Researchers have developed the world’s first human antibody that could inhibit the new coronavirus (SARS-CoV-2) and ‘offers potential for prevention and treatment of COVID-19’. A team of 10 scientists from the University of Utrecht (Utrecht, the Netherlands), the Erasmus Medical Centre (Rotterdam, the Netherlands), and biotech company Harbor BioMed (Cambridge, MA, USA) have published their research online on BioRxiv where it is under peer review before being published by the prestigious journal Nature. More...14 Mar 2020
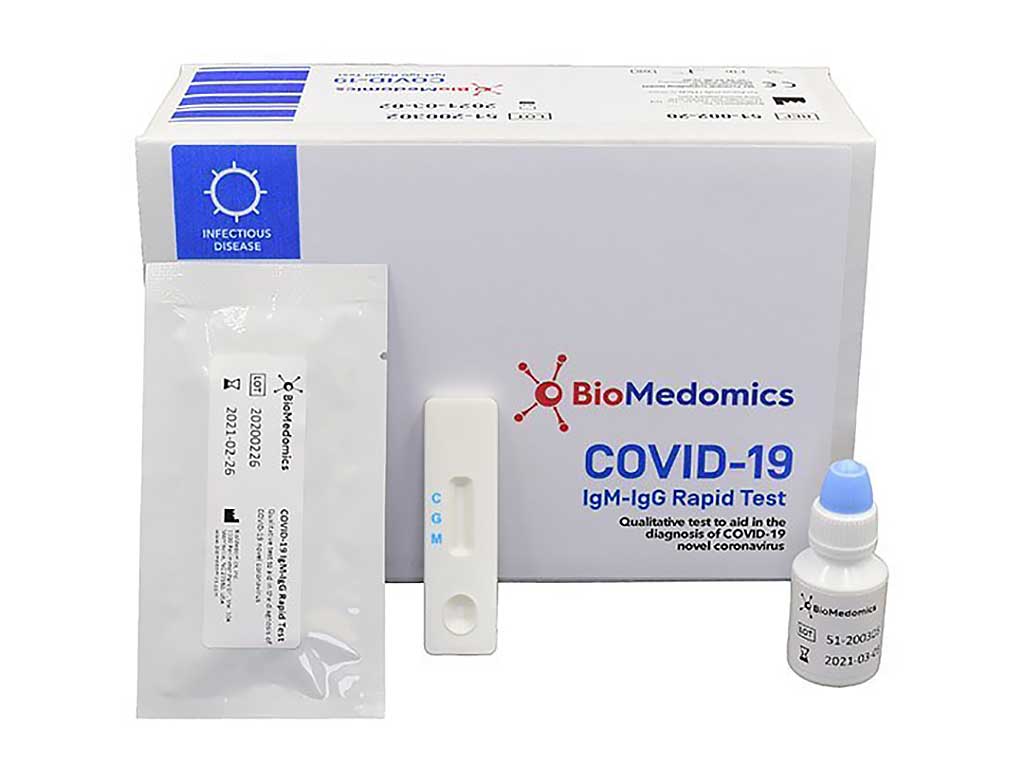
BioMedomics Launches COVID-19 IgM-IgG Rapid Test for Novel Coronavirus
BioMedomics, Inc. (Morrisville, NC, USA) has developed and launched one of the world’s first rapid point-of-care lateral flow immunoassays for the diagnosis of coronavirus infection. The new rapid IgM-IgG combined antibody test for COVID-19 is used to qualitatively detect IgG and IgM antibodies of the novel coronavirus in human serum, plasma or whole blood in vitro. These proteins indicate that a person’s immune system has responded to infection with the COVID-19 virus. It is one of the world’s first rapid tests for coronavirus utilizing this technology. More...14 Mar 2020
Tiziana Life Sciences Accelerate Development of Potential Covid-19 Drug
Tiziana Life Sciences (London, UK), a biotechnology company focused on innovative therapeutics for inflammatory and autoimmune diseases, is expediting development of TZLS-501, a novel, fully human anti-interleukin-6 receptor (anti-IL6R) monoclonal antibody (mAb) for treatment of patients infected with coronavirus COVID-19 (SARS-CoV-2). Tiziana plans to administer TZLS-501 using a proprietary formulation technology. The company has entered into a world-wide license for composition-of-matter of TZLS-501, a fully human mAb targeting IL-6R, with Novimmune, SA, a Swiss biotechnology company in 2017. More...14 Mar 2020
Emergent BioSolutions Collaborates with Novavax for Experimental Vaccine Candidate for Coronavirus Disease
Emergent BioSolutions Inc. (Gaithersburg, MD, USA) has entered into an agreement with Novavax, Inc. (Gaithersburg, MD, USA) whereby Emergent will collaborate with Novavax, utilizing its molecule-to-market contract development and manufacturing (CDMO) services to support bringing into the clinic Novavax’s novel experimental vaccine candidate to protect against coronavirus disease (COVID-19). Under the terms of the agreement, Emergent will produce the COVID-19 experimental vaccine candidate, which is based on the proprietary recombinant protein nanoparticle technology platform of Novavax and utilizing their proprietary Matrix-M™ adjuvant to enhance immune responses. Emergent has initiated work for this program anticipating that the COVID-19 experimental vaccine candidate will be used in a Phase 1 clinical study within the next four months. More...14 Mar 2020
Vir Biotechnology Partners with NIH and Biogen for Developing Coronavirus Antibodies
Vir Biotechnology, Inc. (San Francisco, CA, USA) has entered into a collaboration with Biogen Inc. (Cambridge, MA, USA) for the development and clinical manufacturing of human monoclonal antibodies (mAbs) for the potential treatment of COVID-19, the disease caused by the SARS-CoV-2 virus. More...14 Mar 2020

Hologic’s Panther Fusion System Shows Feasibility for Detection of SARS-Coronavirus-2 (SARS-CoV-2)
Researchers at the Hannover Medical School (Hannover, Germany) have developed a molecular assay for detecting the novel coronavirus SARS-CoV-2 (previously 2019-nCoV) on Hologic, Inc.’s (Marlborough, MA, USA) automated Panther Fusion system. More...10 Mar 2020
In Other News
Global Coronavirus Crisis: Researchers, Industry Mobilize to Offer Remedies (Updated)
Vircell Announces Immediate Release of COVID-19 Research Kit
The Randox Laboratories Diagnostic Test Distinguishes Coronavirus from Other Respiratory Infections
Two Rapid Tests for Diagnosis of the Novel Chinese Coronavirus
CDC Provides Test Protocol for Novel Coronavirus











