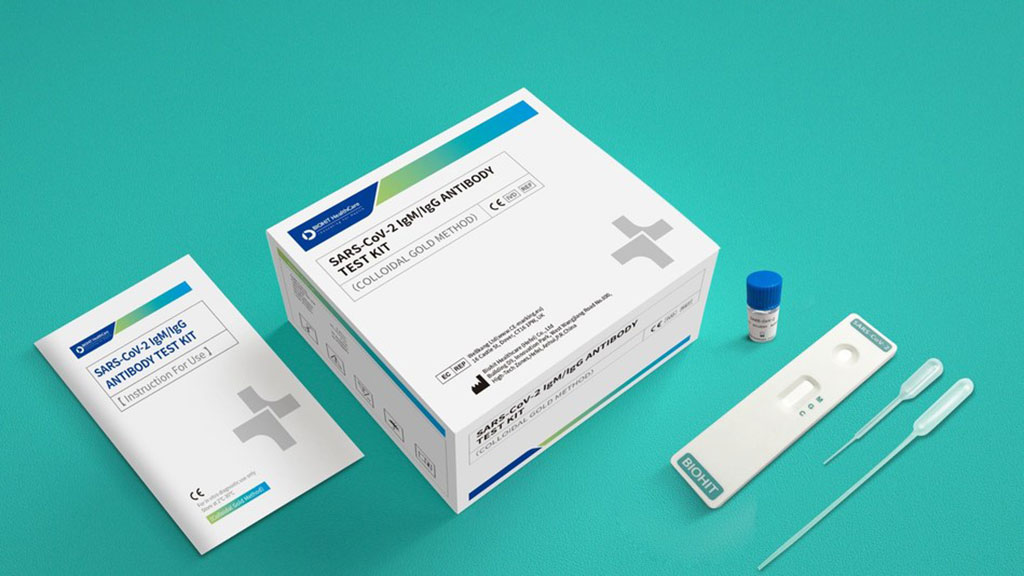
Biohit's SARS-CoV-2 IgM/IgG Antibody Test Kit Granted FDA Emergency Use Authorization
Biohit Healthcare’s (Helsinki, Finland) SARS-CoV-2 IgM/IgG antibody test kit has been authorized by the FDA under a EUA (Emergency Use Authorization) for use in laboratories certified by CLIA (Clinical Laboratory Improvement Amendments of 1988). More...25 Jun 2020



FDA Joins COVID-19 Diagnostics Evidence Accelerator Project
The US Food and Drug Administration (FDA Silver Spring, MD, USA) has announced its participation in the COVID-19 Diagnostics Evidence Accelerator, a multi-stakeholder collaborative project to advance the development of diagnostics. More...23 Jun 2020
In Other News
iPLEX Pro High-Throughput, Low-Cost SARS-CoV-2 Panel Granted Emergency Use Authorization
SpectronRx’s High-Capacity, 1.5 Hour SARS-CoV-2 Test Kit Secures Emergency Use Authorization
CDC Releases Consolidated Recommendations for COVID-19 Testing
PCR Biosystems Launches 4x RT-qPCR Kit for Ultra-Sensitive, High-Throughput Detection of SARS-CoV-2
Ortho Collaborates with BARDA to Accelerate Development of High Specificity SARS-CoV-2 Antibody Tests
Smartphone-Powered, Saliva-Based COVID-19 Diagnostic Test Delivers Lab-Quality Results to App in 20 Minutes
New COVID-19 Test Pinpoints Human Antibodies Specific to Particular Part of SARS-CoV-2 Spike Protein
Beckman Coulter’s SARS-CoV-2 IgG Antibody Test Receives CE Mark
World’s First Quantitative Surrogate Viral Neutralization Test Evaluates COVID-19 Protective Immunity in US
Bead-Assisted Mass Spectrometry (BAMS) Diagnostic Test for COVID-19 Reaches Prototype Stage
First True POC COVID-19 Antibody Tests Offer Convenience and Ease of Use
New POC Device Improves Speed and Accuracy of Lateral Flow Immunoassays to Detect COVID-19 IgM/IgG Antibodies
Cue Health Receives FDA Emergency Use Authorization for Rapid, Molecular POC COVID-19 Test
New Extraction-Free Saliva-Based Test Detects SARS-CoV-2 Without Using Nasal Swab
Awareness Technology Announces Availability of Two-Plate ELISA and Chemiluminescent Analyzer
ChromaCode's High-Throughput HDPCR SARS-CoV-2 Real-Time PCR Assay Granted FDA Emergency Use Authorization
Illumina Receives First FDA Emergency Use Authorization for Sequencing-Based COVID-19 Diagnostic Test
PerkinElmer's Euroimmun SARS-CoV-2 Test Secures FDA Emergency User Authorization
Siemens Secures New FDA Emergency Use Authorizations for Coronavirus Antibody Test
Sekisui’s New OSOM Ultra Plus Flu A&B Test to Help Diagnose Respiratory Infections Amid COVID-19 Pandemic
HHS Announces New Laboratory Data Reporting Guidance for COVID-19 Testing
US FDA Releases Test Performance Data of COVID-19 Antibody and Serology Kits
Puritan Medical Opening New Facility to Double COVID-19 Swab Production











