New Diagnostic Test for Heart Failure Patients Could Lead To Diagnostics and Treatments for COVID-19
Cardiologists at the University of Alberta (Edmonton, Alberta, Canada) have found that a new blood test that reliably predicts outcomes for heart failure patients could lead to new diagnostics and treatments for COVID-19 patients as well. More...20 Aug 2020
10-Minute POC COVID-19 Test Accurately Detects SARS-CoV-2 3C-Protease in Saliva Samples
A new 10-minute rapid point-of-care saliva-based test for detecting active SARS-CoV-2 infections has provided positive proof-of-concept data by achieving analytical performance for detecting active 3C-protease in a rapid visual format. More...20 Aug 2020

New 1-Step COVID-19 PCR Collection Buffer for Viral Detection Assays Reduces Cost and Time Required For SARS-CoV-2 Testing
A novel PCR collection buffer allows for immediate use of the saliva and other sample types on authorized tests, cutting out a significant amount of the required processing effort and time. More...19 Aug 2020

Beckman Coulter and Thermo Fisher to Help HHS Expand US Diagnostic Labs’ COVID-19 Testing Capacity
The US Department of Health and Human Services (HHS) will make combined investments of USD 6.5 million in two commercial diagnostic laboratories to expand their capacity for conducting up to four million additional SARS-CoV-2 tests per month. More...19 Aug 2020

ELITech Group Distributes More Than 3.5 Million COVID-19 Tests and Installs Over 500 PCR Systems Globally
ELITechGroup Molecular Diagnostics (Puteaux, France) has distributed more than 3.5 million SARS-CoV-2 tests and also installed more than 500 ELITe InGenius polymerase chain reaction (PCR) systems, globally, with over a 100 systems placed since the start of the COVID-19 pandemic. More...19 Aug 2020
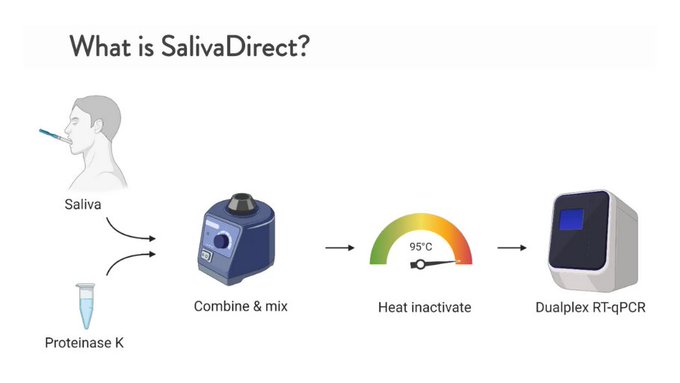
Yale’s SalivaDirect COVID-19 Test with New Method of Saliva Sampling Receives FDA Emergency Use Authorization
The Yale School of Public Health (New Haven, CT, USA) has been issued an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) for its SalivaDirect COVID-19 diagnostic test, which uses a new method of processing saliva samples when testing for COVID-19 infection. More...19 Aug 2020

PerkinElmer Launches Explorer Series Workstations for High-Throughput SARS-CoV-2 Testing With 10,000 COVID-19 Tests Capacity per Day
PerkinElmer, Inc. (Waltham, MA; USA) has launched a series of explorer workstations for SARS-CoV-2 testing capable of preparing and running up to 10,000 COVID-19 tests per day. More...19 Aug 2020
In Other News
AI Spectral Technology COVID-19 Detection Test Achieves 95% Success Rate in Clinical Trial
Panel of Five Serum Biomarkers Identifies COVID-19 Patients at High Risk to Develop Serious Complications
New Low-cost COVID-19 Test Detects Coronavirus in 20 Minutes
Ground-Breaking One-Step COVID-19 Cartridge May Enable Large-Scale Antibody Testing
LumiraDx's SARS-CoV-2 RNA STAR Assay Secures FDA Emergency Use Authorization
Biomeme SARS-CoV-2 Real-Time RT-PCR Test Granted FDA Emergency Use Authorization
New Nasal Swab Robot to Help Expand Testing For SARS-CoV-2 Virus
Hologic Launches Validated Pooling Protocol for COVID-19 Testing
Hologic Launches Validated Pooling Protocol for COVID-19 Testing
Korean Biopharmaceutical Firm Celltrion Launches Both Antigen and Antibody Testing Kits in the US
New COVID-19 Mobile Testing Device Diagnoses Infection Immediately via Breath Sample Using AI
Thermo Fisher Develops New COVID-19 Antibody Assay Targeting Transplant Community
Helix COVID-19 NGS Test One of the First Sequencing-Based COVID-19 Tests To Secure FDA Emergency Use Authorization
BioMérieux SARS-CoV-2 Serology Tests Granted FDA Emergency Use Authorization
New, Highly Sensitive PCR-based COVID-19 Assay Utilizes Switch-Blocker PCR Technology
Eurofins Launches Reagents for Automated Extraction of Genomic SARS-CoV-2 RNA from Clinical Swab Samples
Vela Diagnostics' ViroKey SARS-CoV-2 RT-PCR Test Granted FDA Emergency Use Authorization
UK Government to Roll Out Millions of 90 Minute COVID-19 Test Kits from DnaNudge and Oxford Nanopore
No-Touch, Paper-Based COVID-19 Diagnostic Test Could Detect SARS-CoV-2 Using Electrical Frequencies
Hologic Awarded USD 7.6 Million by HHS and DOD to Ramp Up Capacity for COVID-19 Tests
Eurofins Clinical Diagnostics Launches Lower Cost Highly Accurate COVID-19 PCR Test
Thermo Fisher Launches New High-Throughput, Fully Automated, Real-Time PCR Solution for COVID-19 Testing
Bio-Techne and Mount Sinai Launch Kantaro Quantitative SARS-CoV-2 IgG Antibody RUO Kit










 Analyzer.jpg)
