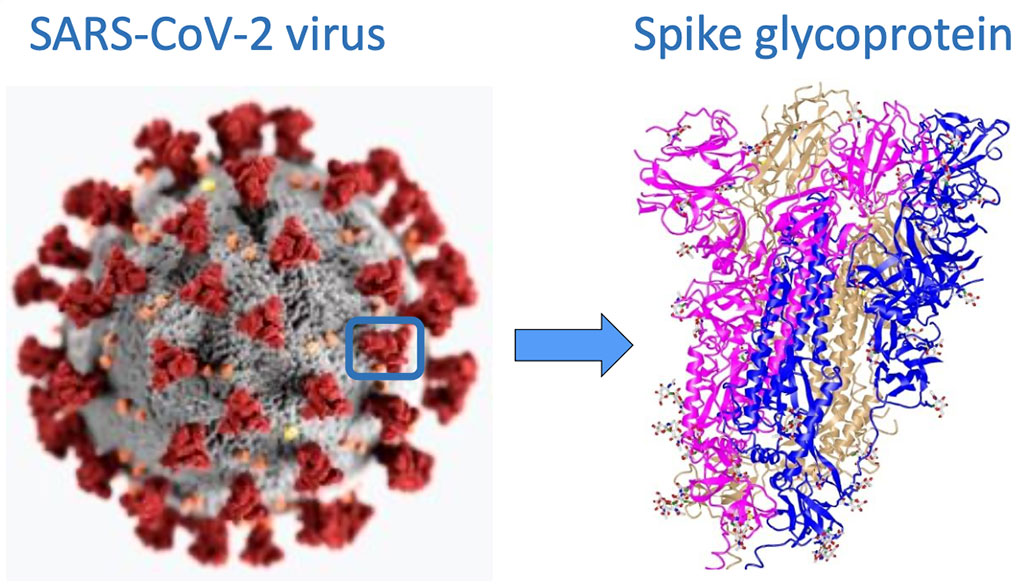
Avacta Launches SARS-CoV-2 Bead-Assisted Mass Spectrometry (BAMS) Research Assay Kit
Avacta Group plc (Wetherby, England) has announced that the BAMS assay to detect the SARS-CoV-2 virus has been launched as a research kit1 by its partner Adeptrix (Beverly, MA, USA) and the assay has been presented by Bruker Scientific (Billerica, MA, USA) in a new application note. More...01 Oct 2020
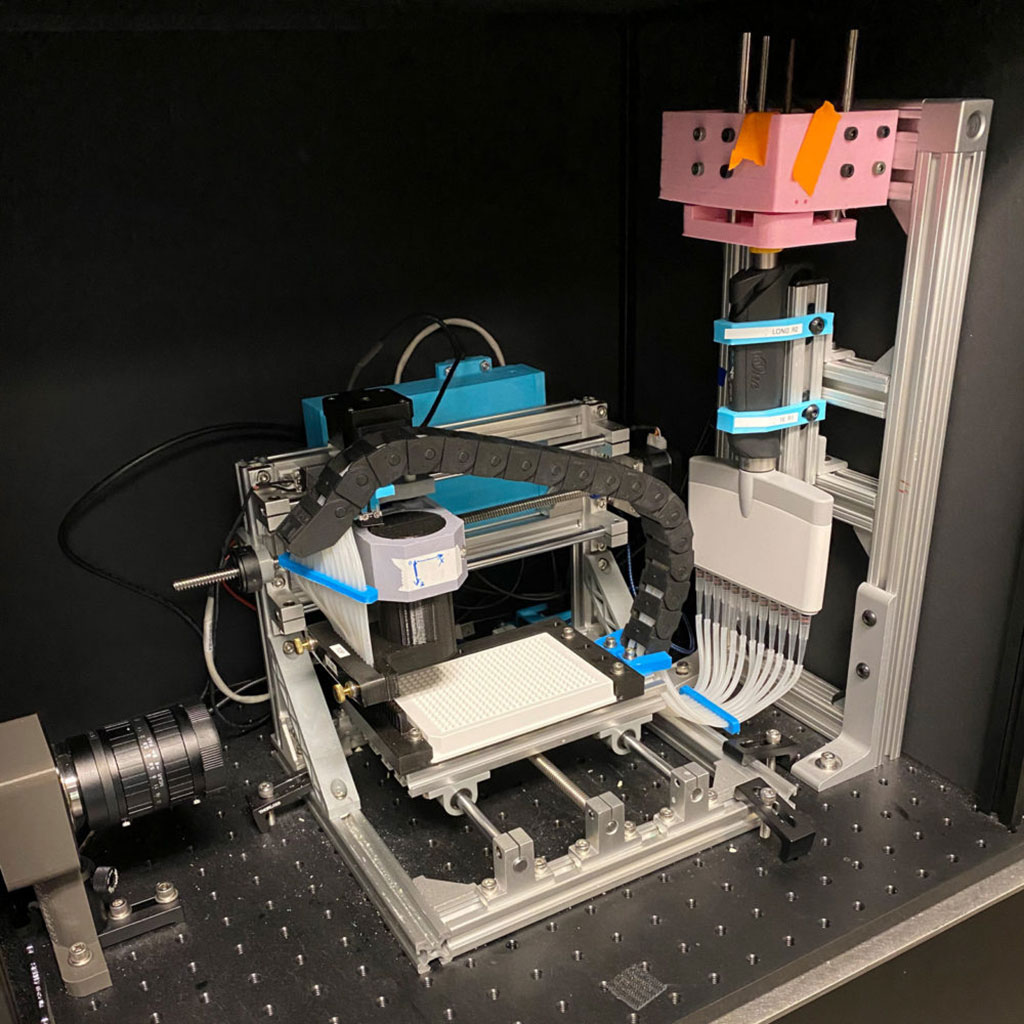
New, Portable Lab-on-a-Chip Identifies Concentration of COVID-19 Antibodies in Human Blood In 15 Minutes
A new, portable lab-on-a-chip can identify the presence of COVID-19 antibodies in blood with greater speed and efficiency than the current standard “enzyme-linked immunosorbent assay” or ELISA technology. More...01 Oct 2020
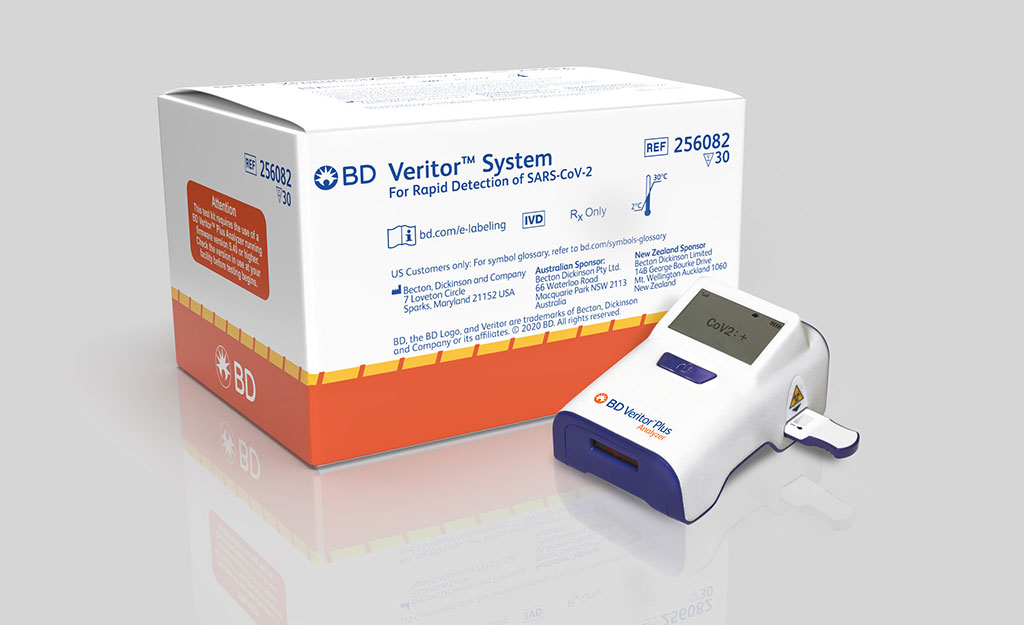
BD Receives CE Mark for Portable, Rapid Point-of-Care COVID-19 Antigen Test That Detects SARS-CoV-2 in 15 Minutes
Becton, Dickinson and Company’s (BD Franklin Lakes, NJ, USA) rapid, point-of-care, SARS-CoV-2 antigen test for use on the BD Veritor Plus System has been CE marked to the IVD Directive (98/79/EC). More...01 Oct 2020
Machine Learning-Based Rapid Diagnostic Reads Immune System to Predict COVID-19 Severity Risk
A rapid diagnostic that reads the immune system to predict severe respiratory failure risk in COVID-19 patients is being developed to help physicians make better hospital admission and resourcing decisions for COVID-19 patients at hospital presentation. More...30 Sep 2020

150 Million Rapid COVID-19 Tests from Abbott Begin Rolling out Across US
The US government has begun distribution of 150 million COVID-19 rapid point-of-care tests from Abbott (Lake Forest, IL, USA), doubling the number of tests that have already been performed across the nation. More...30 Sep 2020
FDA Grants First Ever EUA to COVID-19 POC Antibody Test for Use with Fingerstick Blood Samples
The US Food and Drug Administration (FDA) has issued the first emergency use authorization (EUA) for a serology (antibody) point-of-care (POC) test for COVID-19 using fingerstick blood samples as compared with current approved tests that only utilize serum, plasma, or a venous blood draw. More...30 Sep 2020

120 Million COVID-19 Rapid Tests to Be Made Available for Low and Middle Income Countries
The Access to COVID-19 Tools (ACT) Accelerator has announced a set of agreements to make available affordable, high-quality COVID-19 antigen rapid tests for low and middle-income countries (LMICs). More...30 Sep 2020
In Other News
COVID-19 Saliva Tests Could Detect Silent Carriers
Hologic Granted FDA Emergency Use Authorization for Asymptomatic COVID-19 Testing with Panther Fusion SARS-CoV-2 Assay
Dried Blood Spot Sampling Offers Inexpensive Way to Widen Access to COVID-19 Antibody Testing
Blood-Based Test That Accurately Identifies Viral Infection Before Symptoms Develop Could Help in Early Identification of SARS-CoV-2
New, Portable COVID-19 Saliva-Based Testing Device to Deliver Results in as Little as 20 Minutes
Cepheid Four-in-One Combination Test for SARS-CoV-2, Flu A, Flu B and RSV Granted FDA Emergency Use Authorization
One-Step CRISPR-Based Assay Could Streamline COVID-19 Testing
FDA Authorizes First Nanopore Sequencing-Based Test for SARS-CoV-2
New ABC Test for Influenza A, Influenza B, and COVID-19 to Be Launched in First Week of October
FDA Authorizes First Point-of-Care Antibody COVID-19 Test
Routine Blood Test Predicts Increased Mortality Risk in COVID-19 Patients
New Test Developed with Simplified Buffer Formations Enables Fast, Cheap and Accurate COVID-19 Diagnosis
Four-in-One Test Kit for Detecting Influenza A, Influenza B, RSV and SARS-CoV-2 Set to Launch in November
New COVID-19 Antibody Assay Can Analyze Up to 40,000 Samples per Day Using Serum or Dried Blood
Mannose-Binding Lectin Associated with Coagulopathy in Severe COVID-19
Global COVID-19 Test Kits Market to Surpass USD 13 Billion by 2026
GK Pharmaceuticals Accu-Right SARS-CoV-2 RT-PCR Kit Granted FDA Emergency Use Authorization
World's First Patient-Controlled Nasopharyngeal Swab Robot for COVID-19 Testing Reduces Risk of Exposure to SARS-CoV-2
Ortho Awarded BARDA Contract to Accelerate Development of SARS-CoV-2 Antigen Test
Menarini Diagnostics Launches New SARS-CoV-2 Antigen Test That Also Measures Viral Load
Snibe Diagnostic’s Maglumi 2019-nCoV IgM/IgG Test Granted FDA Emergency Use Authorization
Rapid 90-Minute COVID-19 Test Shows High Accuracy in New Study
Roche Launches New Quantitative Antibody Test to Measure SARS-CoV-2 Antibodies











