Innovative, Label-Free Ratiometric Fluorosensor Enables More Sensitive Viral RNA Detection
Posted on 08 Apr 2025
Viruses present a major global health risk, as demonstrated by recent pandemics, making early detection and identification essential for preventing new outbreaks. While traditional detection methods are effective, they often lack the ability to provide spatiotemporal data on the release of viral genome material. In a significant breakthrough, researchers have developed a novel, label-free ratiometric fluorosensor that selectively and sensitively detects enteroviral RNA. This advancement paves the way for even more efficient detection methods and highlights the importance of interdisciplinary collaboration in addressing global health challenges.
Fluorescent nanoparticles have become powerful tools in bioanalyte sensing, with carbon dots (CDs) leading the charge due to their ease of synthesis, remarkable photostability, tunable photoluminescence, excellent solubility in water, biocompatibility, and versatile surface functionalities for ligand conjugation. These properties make CDs a transformative force in biosensing. At the Nanoscience Center (NSC) at the University of Jyväskylä (Jyväskylä, Finland), researchers have developed an enhanced ratiometric fluorosensor by functionalizing CDs with a Probe (a single-stranded complementary oligonucleotide fragment) and ethidium bromide (EB) for detecting enteroviral RNA. This effort, combining expertise in biology, chemistry, and physics, represents a significant advancement in viral detection technology.
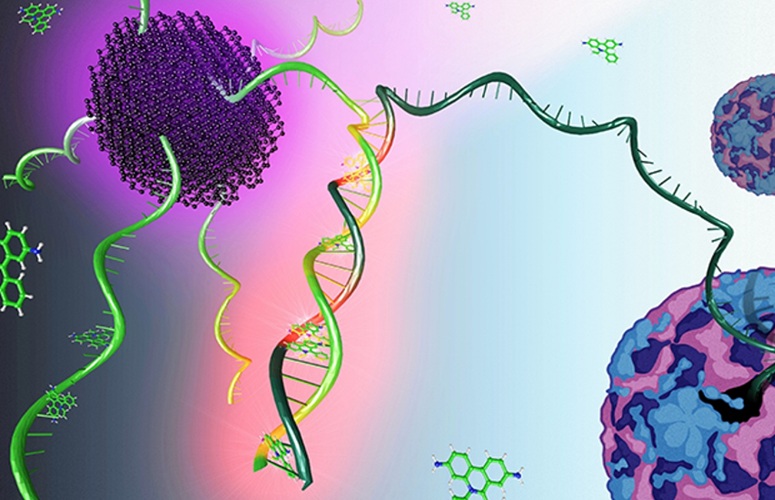
The newly developed Functionalized Sensor (Func Sensor), where the CDs are covalently bonded with the probe, outperforms the traditional Non-Functionalized Sensor (Non-Func Sensor), which is a simple mix of CDs, the probe, and EB. In both sensor types, the target DNA enhances EB fluorescence upon hybridizing with the probe, while the CDs' fluorescence changes slightly due to electron transfer, enabling ratiometric detection. The Non-Func Sensor showed lower sensitivity to target DNA and was ineffective with actual enteroviral RNA samples. In contrast, the Func Sensor demonstrated much higher sensitivity to both DNA and real viral RNA, showing improved selectivity. The improved performance of the Func Sensor is attributed to enhanced charge transfer facilitated by the covalent functionalization of the CDs.
The proof-of-principle study, published in CARBON, emphasizes the significance of covalently immobilizing the probe to improve electron transfer between the CDs and EB, thereby enhancing performance. This research also demonstrates the Func Sensor’s suitability for practical applications in rapid, real-time, and precise in situ detection of viral RNA. Specifically, the study shows that the Func Sensor can detect enteroviral RNA release from the capsid in real time in vitro. This capability positions the Func Sensor as a novel platform for detecting viral RNA during infection, offering a critical advancement in real-time viral RNA detection. The team’s pioneering work not only provides a new method for viral RNA detection but also contributes valuable insights into charge transfer mechanisms between fluorophores. Building on this success, the team is now working to improve the system’s robustness by replacing the potentially harmful ethidium bromide with safer, less cytotoxic, or biocompatible dyes, further enhancing the safety and effectiveness of in vivo viral RNA detection.
Related Links:
University of Jyväskylä








 (3) (1).png)




