Novel Sensor Technology to Enable Early Diagnoses of Metabolic and Cardiovascular Disorders
Posted on 28 Mar 2025
Metabolites are critical compounds that fuel life's essential functions, playing a key role in producing energy, regulating cellular activities, and maintaining the balance of bodily systems. By monitoring these molecules, scientists can gain valuable insights into disease onset, overall health, treatment responses, and the complex operations of biological systems. However, current metabolite sensing techniques have limitations. Most methods rely on resource-heavy lab tests that provide only brief snapshots from isolated samples, while the few continuous sensors available mainly focus on tracking blood sugar levels. Researchers may have now found a way to overcome these limitations.
An interdisciplinary team led by the California NanoSystems Institute at UCLA (CNSI, Los Angeles, CA, USA) has demonstrated a sensor technology based on natural biochemical processes, which can continuously and reliably measure a wide variety of metabolites simultaneously. The researchers see this technology as a complement to traditional lab methods like mass spectrometry, rather than a replacement. Scientists can still use mass spectrometers to identify compounds of interest and then utilize these sensors to monitor those compounds in real-time within living systems. These sensors are built onto electrodes made of tiny cylinders known as single-wall carbon nanotubes. These electrodes act like miniature biochemical laboratories, using enzymes and helper molecules called cofactors to perform reactions that replicate the body's metabolic processes. Depending on the target metabolite, the sensors either detect it directly or convert it into a detectable form via a series of enzymatic reactions. The detection works through enzymes that catalyze electron-exchanging reactions, generating an electrical current on the electrodes’ surface, which can be measured to determine metabolite levels.
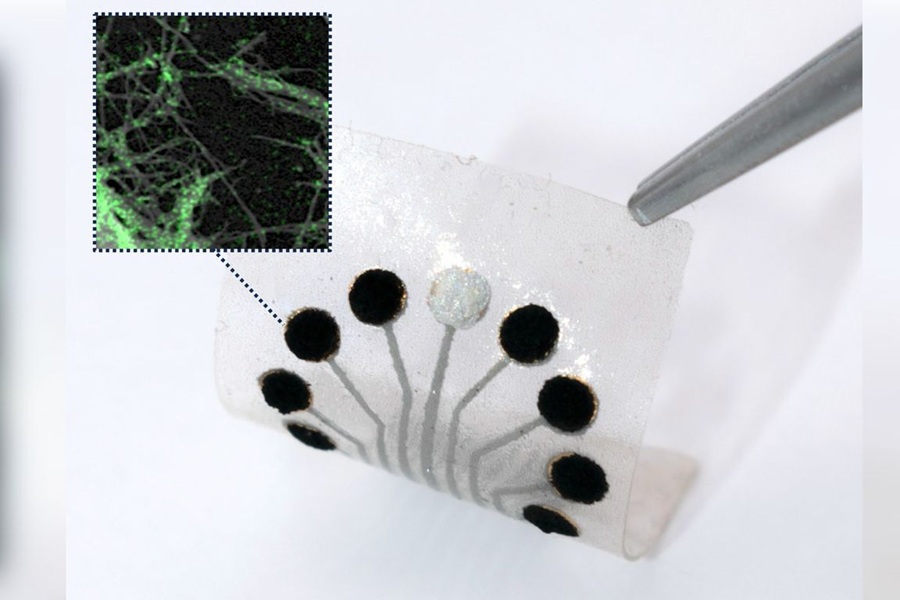
Additionally, other enzymes in the system work to prevent false signals by neutralizing interfering molecules, similar to how enzymes in our bodies detoxify substances. Reflecting the ability to run multiple reactions both in sequence and parallel, the researchers have named this technology "tandem metabolic reaction-based sensors" (TMR sensors). Traditional enzymatic sensors typically support single-step reactions without cofactors, but by incorporating cofactors, TMR sensors can detect over 800 metabolites and cover more than two-thirds of the body’s metabolites with just one conversion step. In a series of experiments, the team showed that the technology could continuously and sensitively measure a set of 12 clinically important metabolites. They successfully measured metabolites in sweat and saliva from patients undergoing treatment for epilepsy and from individuals with conditions resembling diabetes complications. The study, published in Proceedings of the National Academy of Sciences, also revealed a gut bacteria-derived metabolite in the brain that could cause neurological disorders if it accumulates.
The ability of these sensors to track a broad range of metabolites across various biological settings presents exciting possibilities for advancing human health and scientific research. These sensors could transform the care of metabolic and cardiovascular diseases by enabling earlier and more precise diagnoses, as well as tailoring treatments to individual metabolic profiles. The technology could also optimize fitness and athletic performance by tracking how the body metabolizes energy under different conditions. In drug development, these sensors could provide real-time insights into how treatments influence metabolic pathways — from evaluating cancer therapies that inhibit tumor growth through enzyme inhibition to monitoring bacterial metabolite production for better-targeted antibiotics. With many potential applications, this technology also holds promise in exploring the gut-brain connection — a burgeoning area of biomedical research. The research team is now working to adapt their platform to address unresolved research questions and explore new diagnostic opportunities.
“Decades of research have mapped natural metabolic pathways linking metabolites to specific enzymatic reactions,” said senior corresponding author Sam Emaminejad, an associate professor of electrical and computer engineering at the UCLA Samueli School of Engineering and a CNSI member. “By adapting carefully selected enzymes and cofactors for different functions, our electrodes replicate these complex reactions, enabling reliable detection of a far broader set of metabolites than conventional sensors. The robustness comes from evolution itself — enzymes and cofactors, refined over tens of millions of years, are highly sensitive, specific, and stable. We’re harnessing nature’s own blueprint and molecular machinery to track the very biochemical processes they sustain.”
Related Links:
CNSI














