Low-Cost Portable Sensor Detects Heavy Metals in Sweat
Posted on 24 Jan 2023
Heavy metals like lead and cadmium can be found in batteries, cosmetics, food and many other things that have become a part of daily life. However, they become toxic if they accumulate in the human body and can cause several health problems. Expensive equipment and a controlled laboratory environment are usually required to detect heavy metals in body fluids. Researchers have now developed a portable sensor made of simple materials that can detect heavy metals in sweat, which is easily sampled.
Humans discharge heavy metals mostly through sweat and urine. The analysis of these biofluids forms a key part of toxicological tests as well as treatment. As compared to other gold-standard tests for detecting heavy metals in biofluids, the new sensor developed by researchers at the University of São Paulo (São Paulo, Brazil) is simple in terms of the materials used to manufacture it and the production stages it goes through. The device is connected to a potentiostat, a portable instrument that determines the concentration of each metal by measuring differences in potential and current between electrodes. The result is displayed on a computer or smartphone using appropriate application software. The system is so simple that it can be used by non-specialists without training, as well as by technicians in hospitals, clinics and doctor’s offices.
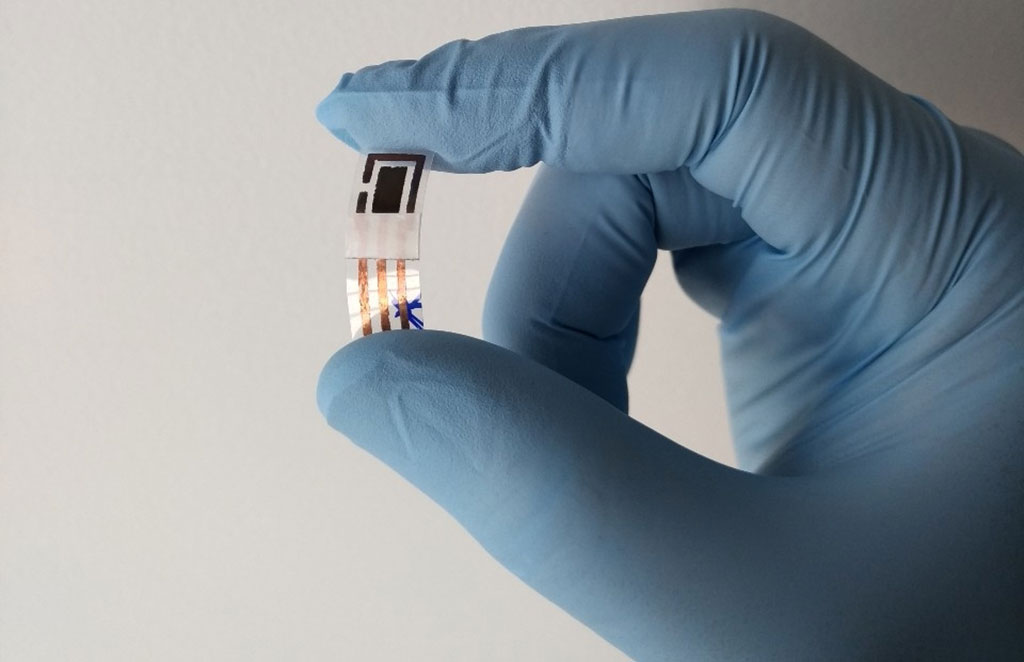
“We get important information on a person’s health by measuring their exposure to heavy metals. High levels of cadmium can lead to fatal problems in the airways, liver and kidneys. Lead poisoning damages the central nervous system and causes irritability, cognitive impairment, fatigue, infertility, high blood pressure in adults and delayed growth and development in children,” said Paulo Augusto Raymundo Pereira, last author of the article. “The world needs flexible sensors that are easily, cheaply and rapidly mass-produced, as our device is, for on-site detection, continuous monitoring and decentralized analysis of hazardous compounds.”
“The base of the device is polyethylene terephthalate (PET), on top of which is a conductive flexible copper adhesive tape, a label of the kind you can buy from a stationer’s, with the sensor printed on it, and a protective layer of nail varnish or spray,” said Robson R. da Silva, co-author of the article. “The exposed copper is removed by immersion in ferric chloride solution for 20 minutes, followed by washing in distilled water to promote the necessary corrosion. All this ensures speed, scalability, low power and low cost.”
Related Links:
University of São Paulo














