Rapid COVID Test Developed Solves Problems With LAMP Sensitivity
Posted on 14 Feb 2022
Researchers from the University of California, Santa Barbara have developed an isothermal amplification-based SARS-CoV-2 and influenza test that could retail for as little as $2 while addressing a key problem with loop-mediated isothermal amplification tests — false positive results.
Loop-mediated isothermal amplification (LAMP) diagnostics have gained attention for pathogen detection because they do not require sophisticated, expensive instrumentation or highly trained personnel for operation. Five principal tenets of point-of-care (POC) clinical diagnostics include speed, sensitivity, affordability, scalability, and accessibility, without a concomitant need of specialized and costly equipment.
Numerous methods for the detection of SARS-CoV-2 virus, including molecular, antigen, and serology tests, are currently in use. Although molecular methods such as polymerase chain reaction (PCR) are rapid and sensitive, they generally require access to specialized and costly laboratory instrumentation and highly trained personnel, and they are technologically complex for POC applications or resource-limited settings.
Molecular Biotechnologists at the University of California, Santa Barbara (Santa Barbara, CA, USA) and their colleagues enrolled two subgroups of participants (symptomatic and asymptomatic) at Santa Barbara Cottage Hospital (Santa Barbara, CA, USA). The symptomatic group consisted of 20 recruited patients who tested positive for SARS-CoV-2 with symptoms; 30 asymptomatic patients were recruited from the same community, through negative admission screening tests for SARS-CoV-2. Among the 50 eligible participants with no prior SARS-CoV-2 infection included in the study, 29 were men. The mean age was 57 years (range, 21 to 93 years).
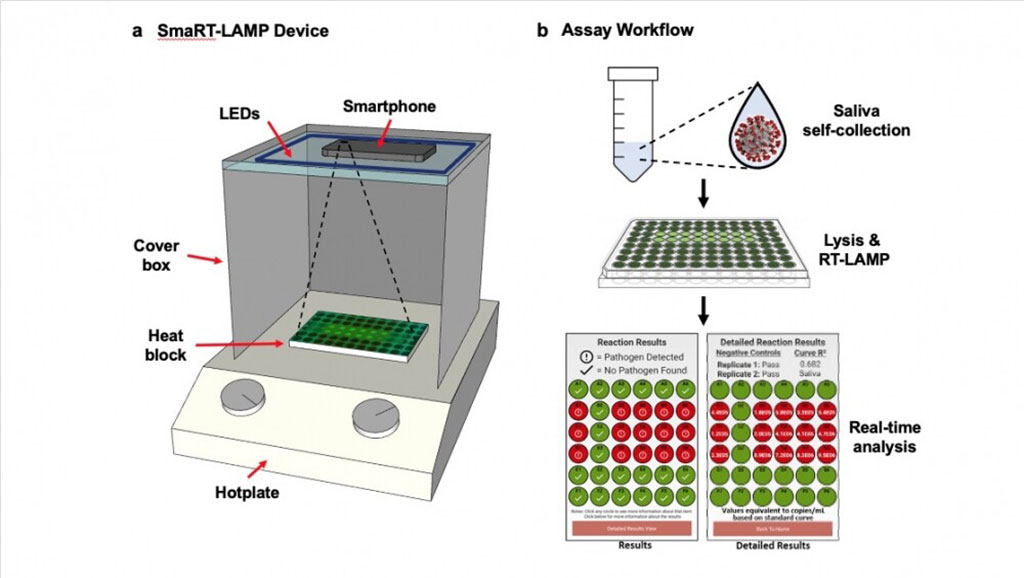
The smartphone-based real-time loop-mediated isothermal amplification (smaRT-LAMP) was first optimized for analysis of human saliva samples spiked with either SARS-CoV-2 or influenza A or B virus. These results then were compared with those obtained by side-by-side analysis of spiked samples using the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) criterion-standard reverse transcriptase–quantitative polymerase chain reaction (RT-qPCR) assay. Next, both assays were used to test for SARS-CoV-2 and influenza viruses present in blinded clinical saliva samples obtained from 50 hospitalized patients.
The investigators reported that SmaRT-LAMP exhibited 100% concordance (50/50 patient samples) with the CDC criterion-standard diagnostic for SARS-CoV-2 sensitivity (20/20 positive and 30/30 negative) and for quantitative detection of viral load. This platform also met the CDC criterion standard for detection of clinically similar influenza A and B viruses in 20 spiked saliva samples, and in saliva samples from hospitalized patients (50/50 negative). The smartphone-based LAMP assay was rapid (25 minutes), sensitive (1,000 copies/mL), low-cost (< USD 7/test), and scalable (96 samples/phone).
The authors concluded that the smartphone-based LAMP assay integrates reliable diagnostics with advantages of smartphone detection, offering an inexpensive diagnostic platform for SARS-CoV-2 and influenza A and B viruses that match the CDC RT-qPCR criterion standards. The study was published on January 28, 2022 in the journal JAMA Network Open.
Related Links:
University of California, Santa Barbara
Santa Barbara Cottage Hospital
Centers for Disease Control and Prevention