Use of Dried Blood Samples Facilitates Tracking of COVID-19 Antibodies
By LabMedica International staff writers
Posted on 13 Oct 2020
A recent study confirmed that dried blood spot samples could be used as an alternative to venous blood for detecting antibodies to the SARS-CoV-2 virus (COVID-19).Posted on 13 Oct 2020
A confirmed diagnosis of COVID-19 depends on the detection of RNA from the coronavirus. In contrast, while serological testing is less useful for diagnosing the acute stages of infection, it is vitall for determining prior virus exposure at a population level.
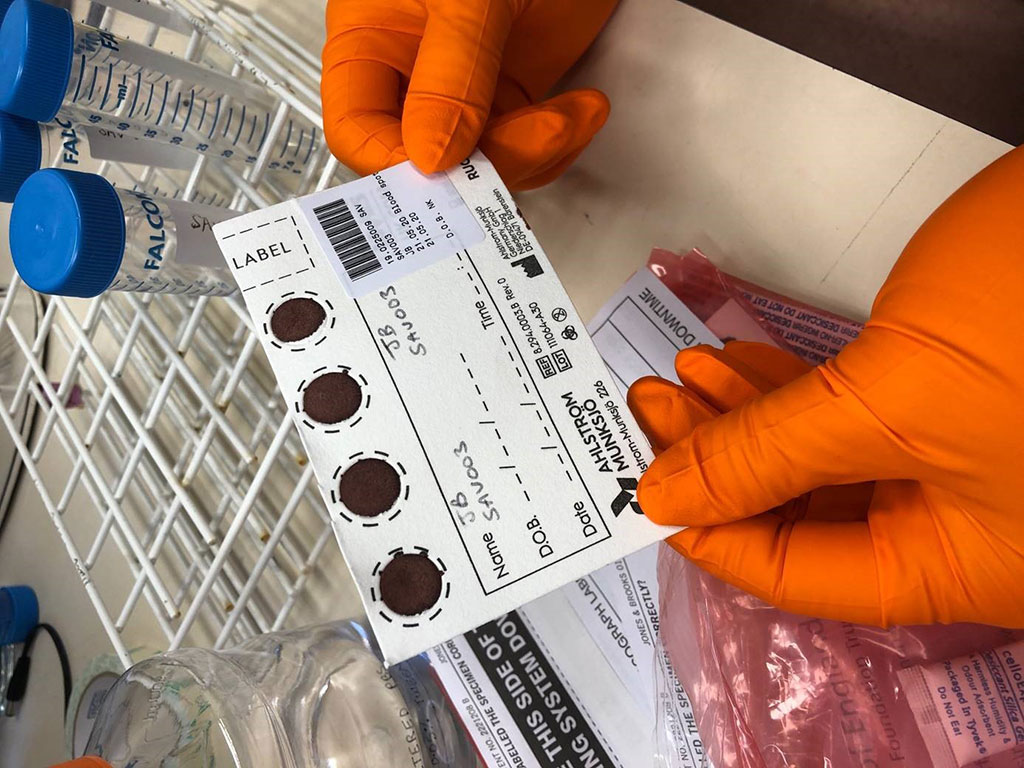
Image: DBS sampling is simple, inexpensive and can be self-collected by the patient at home, using a simple finger prick (Photo courtesy of University of Birmingham)
Currently, antibody testing for COVID-19 uses serum or plasma collected by venipuncture. The use of such sampling in large-scale seroepidemiologic studies is limited by logistic challenges, resources, and costs. In contrast, dried blood spot (DBS) sampling, which is a well-established method for preserving antibodies against various infections, is simple, inexpensive, and can be self-collected and then sent by postal services to laboratories for processing.
Up to now, the potential role of DBS sampling for studying the prevalence of SARS-CoV-2 antibodies has not been fully explored, and knowledge regarding the recovery of such antibodies from the DBS is limited.
To improve this situation, investigators at the University of Birmingham (United Kingdom) described the validation of DBS samples against matched serum in a highly sensitive and specific SARS-CoV-2 ELISA.
The investigators analyzed serum and DBS samples from volunteers at University Hospitals Birmingham Foundation NHS Trust, some of whom had previously tested positive for SARS-CoV-2 by molecular tests, while the status of other volunteers was either negative or unknown. Results revealed that relative to serum samples, DBS samples achieved 98% sensitivity and 100% specificity for detecting anti-SARS-CoV-2 S glycoprotein antibodies. Furthermore, 100% of PCR-positive samples were also antibody-positive in DBS.
Senior author Dr. Matthew O'Shea, a researcher in the institute of immunology and immunotherapy at the University of Birmingham, said, "Our results have demonstrated that dry blood spot sampling not only offers a viable alternative for antibodies testing, but one that overcomes the limitations that current methods can present by eliminating the need for skilled phlebotomists. DBS offers the opportunity for wider population-level testing and improved surveillance in vulnerable groups such as patients with chronic conditions, the immunocompromised, and the elderly by removing the need to come into contact with a healthcare professional during sample collection."
The study was published in the December 2020 online edition of the journal Emerging Infectious Diseases.
Related Links:
University of Birmingham