Circulating Exosomes Analysis Uses Microfluidic Technology
By LabMedica International staff writers
Posted on 23 Oct 2014
A new microfluidic approach has been developed to streamline and expedite the exosome analysis pipeline by integrating specific immunoisolation and targeted protein analysis of circulating exosomes.Posted on 23 Oct 2014
Tumor-derived exosomes have attracted increasing interest in non-invasive cancer diagnosis and monitoring of treatment response which will help in developing blood-based tests for disease diagnosis, especially when biopsy is difficult, costly, and sometimes not even an option.
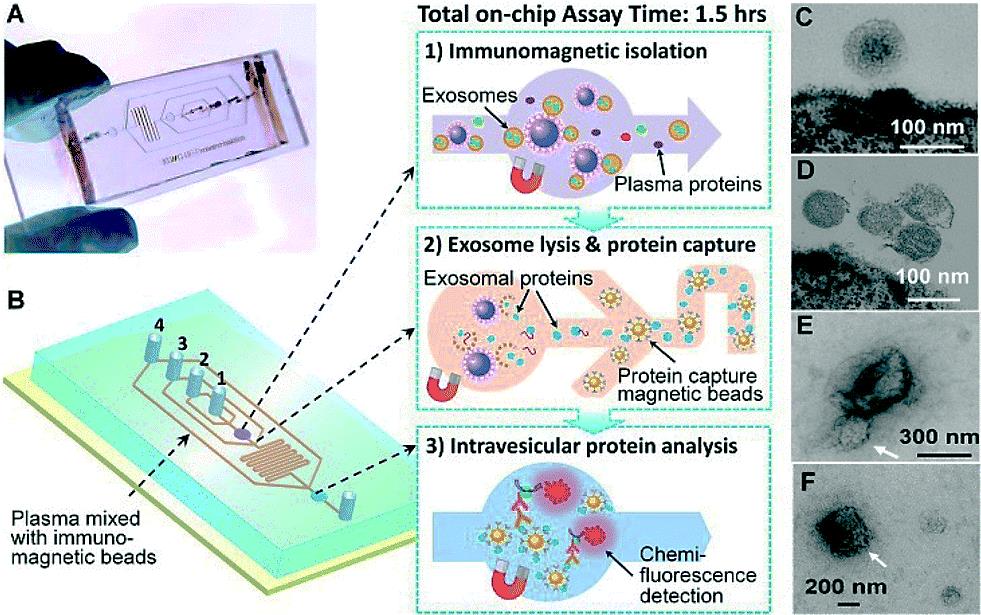
Image: The prototype polydimethylsiloxane (PDMS) chip containing a cascading microchannel network for multi-stage exosome analysis (Photo courtesy of the University of Kansas).
Scientists and bioengineers at the University of Kansas Medical Center (Kansas City, KS, USA) devised an approach that enables selective subpopulation isolation and quantitative detection of surface and intravesicular biomarkers. This can be done directly from a minimally invasive amount of plasma samples, within about 100 minutes with markedly improved detection sensitivity. The microfluidic technology uses a magnetic bead-based strategy to integrate and streamline the multi-step analysis of exosomes directly from human plasma. Compared to the surface-based exosome microchips the immunomagnetic method allows for enrichment of captured exosomes and convenient sample preparation for transmission electron microscopy (TEM) characterization in addition to higher capture efficiency and analysis sensitivity due to the larger surface area.
The scientists applied the technology to analyze clinical plasma specimens, mainly from non-small-cell lung cancer (NSCLC) patients. The prototype lab-on-a-chip is made of a widely used silicone rubber called polydimethylsiloxane and uses a technique called "on-chip immunoisolation”. To demonstrate the ability to detect exosomal expression patterns associated with cancer, the team conducted relative quantification of five exosome subpopulations defined by individual surface markers using TEM. They optimized the on-chip bead-based immunoassay and chemifluorescence readout using a matched pair of capture/detection antibodies, an alkaline phosphatase (AP)-conjugated secondary antibody, and the 6,8-Difluoro-7-Hydroxy-4-Methylcoumarin (DiFMUP) substrate.
Yong Zeng, PhD, a co-author of the study, said, “Our technique provides a general platform to detecting tumor-derived exosomes for cancer diagnosis. In addition to lung cancer, we've also tested for ovarian cancer in this work. In theory, it should be applicable to other types of cancer. Our long-term goal is to translate this technology into clinical investigation of the pathological implication of exosomes in tumor development. Such knowledge would help develop better predictive biomarkers and more efficient targeted therapy to improve the clinical outcome.” The study was published in the October 2014 issue of the journal Lab on a Chip.
Related Links:
University of Kansas Medical Center









 Analyzer.jpg)