Pioneering Microscopy Technique Improves Diagnosis of Glioblastoma Brain Tumors
Posted on 16 Oct 2024
Along the brain’s largest nerve fiber highway, known as the corpus callosum, travel cells that form one of the most lethal brain cancers, glioblastomas. Now, scientists have developed a cellular detector by integrating artificial intelligence (AI) with a cutting-edge microscope, enabling them to visualize and monitor specific cells with unprecedented clarity in deep brain tissue, including along this superhighway. The researchers believe that understanding the early ‘traffic patterns’ of cancer cells along the corpus callosum could aid in establishing a biomarker for the earlier detection of glioblastomas in patients, potentially enhancing future diagnostic tools.
This collaborative endeavor between EMBL (Heidelberg, Germany) and Heidelberg University (Heidelberg, Germany) builds upon a new microscopy technique developed by EMBL researchers in 2021 in association with colleagues from Germany, Austria, Argentina, China, France, the United States, India, and Jordan. The EMBL team worked with these diverse partners to tackle some of the challenges neuroscientists encounter when studying deep brain regions. Previously, the diffuse nature of brain tissue made it challenging for scientists to observe neurons and glial cells, such as astrocytes, and to investigate their communication within the cortex. This difficulty extended to visualizing neural cells in the hippocampus, another deep brain area critical for spatial memory and navigation. The researchers' new method utilized advanced microscopy techniques that offered a broader and clearer view while compensating for the distortion caused by scattered light waves in deep brain tissue.
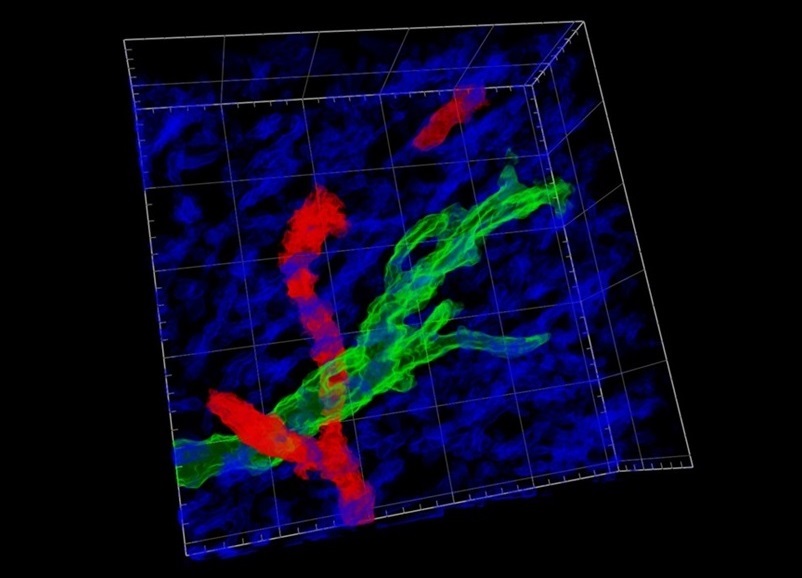
Now, in a study published in the journal Nature Communications, the EMBL researchers collaborated with neuroscientists, neurooncologists, and AI specialists to enhance this microscope further. The outcome is a device capable of observing living neurons and other brain cell types deep within the brain over extended periods. Glioblastomas are predominantly a white-matter disease. The new advanced imaging technique allowed the team to study these tumor cells within their microenvironment in the white matter. This capability was vital for understanding how tumor cells invade the densely myelinated (insulated) fiber “lanes” of the corpus callosum and subsequently adapt and spread throughout the brain. This invasion is also linked to the critical structures of the brain that glioblastomas invade in a lethal manner.
A key aspect of this recent collaboration was the integration of AI, which helped to reduce noise in the images, resulting in much clearer contrast. The AI can differentiate various structures within the white matter, such as myelinated fibers and blood vessels, which is significant for multiple reasons. A tailored workflow enabled the researchers to separate blood vessel signals from those of the myelinated neural fibers, clarifying the microenvironment of the tumor cells. As a result, the researchers identified a potential microscopic imaging biomarker associated with the structural characteristics of the white matter microenvironment. This innovative workflow paves the way for identifying imaging patterns for glioblastomas, allowing for earlier detection than currently possible. Their next steps involve integrating additional advanced imaging modalities to create practical tools for standard clinical applications.
“These findings also help explain the current challenges in detecting glioblastoma cells at the tumor’s infiltrative edges using conventional MRI techniques, which are the standard in clinical imaging,” said Varun Venkataramani at the Neurology Clinic of the University Hospital Heidelberg. “As a neuroscientist, neurologist, and neurooncologist, I see potential for this technology to bridge the gap between laboratory research and clinical application, improving how we could diagnose and potentially treat brain tumors.”














