World’s First Precision Medicine Test Predicts Progression to Esophageal Cancer in Patients with Barrett’s Esophagus
Posted on 17 Oct 2022
Esophageal adenocarcinoma (EAC) is one of the fastest-growing cancers (by incidence) in the world and is associated with poor outcomes. Chronic gastrointestinal reflux (GERD) is a major risk factor for the development of EAC. Chronic reflux in the esophagus causes changes to the molecular and cellular features of the esophagus, which often results in a condition called Barrett’s Esophagus (BE). BE is a serious complication of GERD and a risk factor for the development of EAC. Today, gastroenterologists cannot adequately determine which patients with BE will progress to esophageal cancer. As a result, patients with BE must undergo regular surveillance endoscopies to check the status of the condition. New diagnostic and prognostic tests are needed to provide actionable information to physicians and patients managing BE.
Until now, there has been no adequate way to determine the risk level of patients with BE. There is no single biomarker that can sufficiently diagnose the grade of dysplasia or predict malignant progression since multiple pathways play a role in disease progression. As a result, many high-risk patients do not receive the intervention they need, while low-risk patients have undergone unnecessary surveillance endoscopies and experienced needless anxiety about developing EAC. Now, new data from a randomized controlled trial (RCT) has shown that a precision medicine test can significantly improve a physician’s accuracy in assessing the risk of progression to high-grade dysplasia (HGD) or EAC in patients diagnosed with BE.
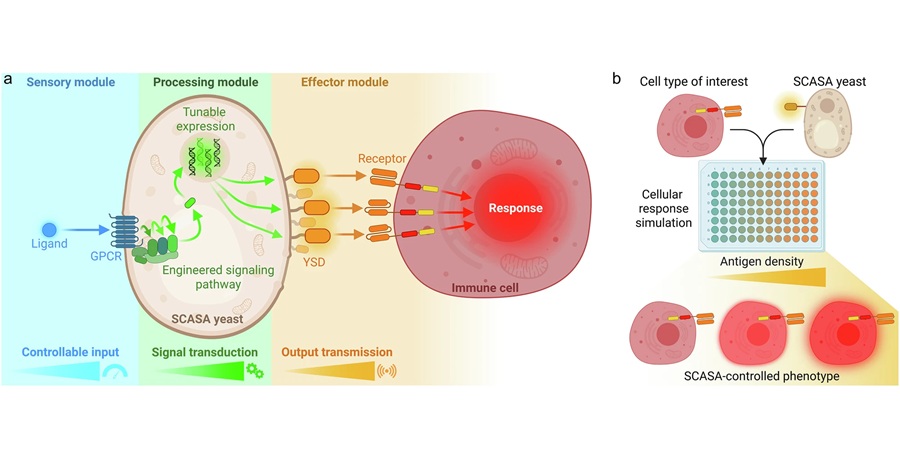
TissueCypher is a precision medicine test from Castle Biosciences (Pittsburgh, PA, USA) that is designed to predict progression to HGD and/or EAC within five years for patients diagnosed with BE. TissueCypher is indicated for use in patients with endoscopic biopsy confirmed BE that is graded non-dysplastic (ND), indefinite for dysplasia (IND) or low-grade dysplasia (LGD). In the RCT, 259 physicians were randomized to three groups and asked to evaluate clinical performance and value (CPV) vignettes with high- and low-risk patient scenarios based on clinical risk factors. A quality-of-care percentage (0-100%) score was generated from the CPVs based on the American College of Gastroenterology (ACG) and the American Society of Gastrointestinal Endoscopy (ASGE) guidelines. Quality-of-care scores improved significantly across all patient cases after physicians were given the TissueCypher test results.
“The study data showed that participants who ordered or received TissueCypher test results were up to 65.6% more likely to predict progression to HGD or EAC, (p<0.001), when compared to physicians who did not receive TissueCypher test results in our randomized trial,” noted John W. Peabody, M.D., Ph.D., first study author. “Importantly, subsequent to receiving test results and making their assessment, the intervention group was also more likely to adhere to guideline-recommended management strategies.”
“Barrett’s esophagus remains a persistent and real-world clinical challenge for endoscopists and patients. Individuals with non-dysplastic Barrett’s esophagus constitute the vast majority of cases, and for years, we have seen few updates in the management strategy of this patient population in particular,” said Craig Munroe, M.D., gastroenterology medical director at Castle Biosciences. “We were very excited to present the results of the QURE study, which help further demonstrate TissueCypher's potential to meaningfully advance the care of this important patient population. We believe the clinical utility and objective information provided by our test can equip physicians with the information they need to make more informed treatment plan decisions and move beyond the limitations in the current standard of care for risk stratification of patients with BE.”
Related Links:
Castle Biosciences








 (3) (1).png)