Digital Cell Morphology Platform May Replace Microscopes
Posted on 15 Jun 2022
With the increasing worldwide prevalence of hematological disorders and malignancies, the accuracy and timeliness of Peripheral Blood Smear (PBS) results are critically important for early diagnosis and effective treatment initiation. The PBS review is a powerful diagnostic tool that provides rapid, reliable access to information about a variety of disorders such as blood related cancers, anemia, infections, and allergies. The majority of these tests are performed manually, but now a disruptive PBS morphology solution completely eliminates the need for additional manual microscopic examination.
Scopio Labs’ (Tel Aviv, Israel) ground-breaking X100HT device with PBS application combines high throughput capabilities with the highest resolution for hematological analysis, catering to major medical facilities and labs across the world. In order to overcome the problem of critical shortage of medical laboratory professionals, Scopio delivers end to end digital transformation of the lab process for Full-Field PBS review of white blood cells, red blood cells, and platelet estimation. The results of every assessment (including images, annotations and flagged abnormalities) are automatically documented in a standardized digital report and seamlessly shared across the continuum of care.
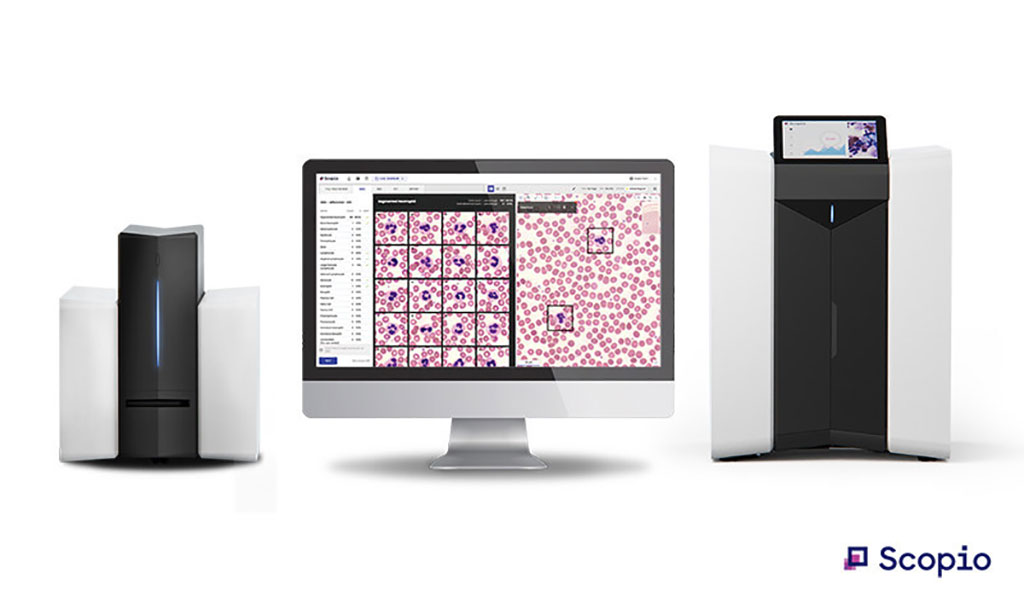
In manual microscopy, users must choose between either a large field of view or high resolution. Scopio's devices eliminate that tradeoff completely, capturing large scan areas at 100X magnification. Holding 30 slides and processing up to 40 samples an hour, Scopio's X100HT can meet the high throughput requirements of large hospitals and labs, while fully supporting remote review capabilities, enabling a new subfield of telehematology. Hospital and lab networks can now operate seamlessly across multiple facilities of all sizes, with workload balancing, remote consultations, addressing personnel shortages and more. Scopio Labs has received U.S. FDA 510(k) clearance for its X100HT device with PBS Application, marking the company's second FDA clearance and broadening its suite of fully digital platforms to cater to all lab sizes and networks.
"We're excited to expand our suite of fully digital AI-powered diagnostic platforms to accelerate PBS analysis, improve consistency of results, and reduce review time," said Erez Naaman, CTO and Co-Founder of Scopio Labs. "At Scopio, we are determined to usher in the digital revolution to laboratory medicine. Our devices offer complete remote capabilities for real-time diagnosis and treatment decisions, allowing experts to review, collaborate, and consult from anywhere, and at any time, using our AI-powered applications."
Related Links:
Scopio Labs














