Blood-Based MicroRNA Signatures Distinguishes Individuals with Lung Cancer
By LabMedica International staff writers
Posted on 16 Mar 2020
The overall low survival rate of patients with lung cancer calls for improved detection tools to enable better treatment options and improved patient outcomes. Lung cancer affects about 228,000 people a year in the USA and has a five-year survival rate just shy of 20%. Posted on 16 Mar 2020
Multivariable molecular signatures, such as blood-borne microRNA (miRNA) signatures, may have high rates of sensitivity and specificity but require additional studies with large cohorts and standardized measurements to confirm the generalizability of miRNA signatures. MicroRNA signatures appear to distinguish individuals with lung cancer from those with other lung diseases as well as from those without a lung condition.
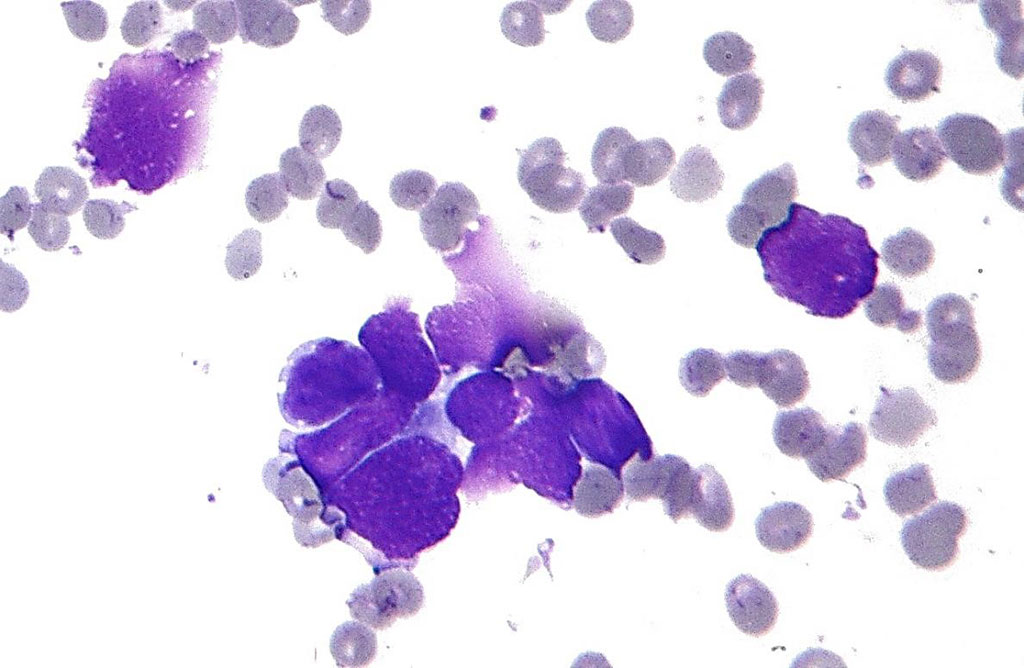
Image: Histopathology showing the key features of small cell lung carcinoma (SCLC): Nuclear molding; salt and pepper chromatin; and scant cytoplasm (Photo courtesy of Nephron).
A large team of scientists collaborating with Saarland University (Saarbrücken, Germany) investigated the use of blood-borne miRNAs as potential circulating markers for detecting lung cancer in an extended cohort of symptomatic patients and control participants. Clinical diagnoses were obtained for 3,046 patients (606 patients with non–small cell and small cell lung cancer, 593 patients with non-tumor lung diseases, 883 patients with diseases not affecting the lung, and 964 unaffected control participants). The team calculated the sensitivity and specificity of liquid biopsy using miRNA signatures for detection of lung cancer. Blood samples collected from the participants underwent genome-wide miRNA profiling using human miRNA microarrays.
The investigators split their cohort into equal-sized training and validation sets. Within the training set, they uncovered a 15-miRNA signature that could distinguish patients with lung cancer from all other individuals. In the validation set, this signature could diagnose lung cancer with an accuracy of 91.4%, a sensitivity of 82.8%, and a specificity of 93.5%. Similarly, they uncovered a 14-miRNA signature that could distinguish patients with lung cancer from those with a non-tumor lung disease with 92.5% accuracy, 96.4% sensitivity, and 88.6% specificity. A third signature of 14 miRNAs could distinguish patients with early-stage lung cancer from all other patients with an accuracy of 95.9%, a sensitivity of 76.3%, and a specificity of 97.5%. Although the team focused on general lung cancer biomarkers, they noted that the miRNA hsa-miR-30a-5p was best able to tell small cell lung cancer and non-small cell lung cancer apart.
The authors concluded that their study suggested that the identified patterns of miRNAs may be used as a component of a minimally invasive lung cancer test, complementing imaging, sputum cytology, and biopsy tests. The study was published on March 5, 2020 in the journal JAMA Oncology.
Related Links:
Saarland University











 (3) (1).png)


