Microfluidic Device Isolates Circulating Tumor Cell Clusters
By LabMedica International staff writers
Posted on 03 Jul 2019
The three main challenges of cancer treatment are metastases, recurrence, and acquired therapy resistance. These challenges have been closely linked to circulating cancer cell clusters.Posted on 03 Jul 2019
About 90% of cancer deaths are due to metastases, when tumors spread to other vital organs, and it has recently been realized that it's not individual cells but rather distinct clusters of cancer cells that circulate and metastasize to other organs.
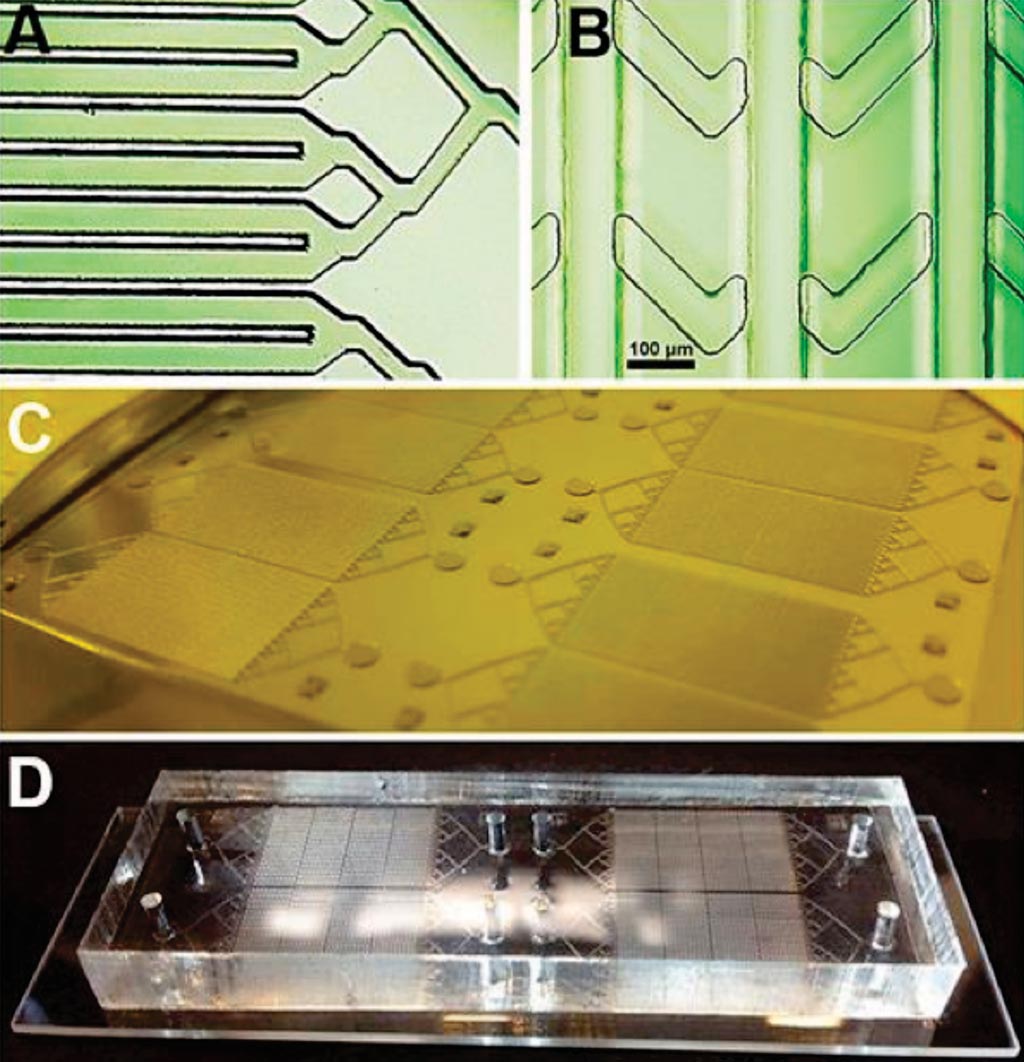
Image: (A and B) Photomicrographs of the layers of the device; (C) the mold ready for casting and (D) the chip mounted on a slide (Photo courtesy of San Diego State University).
A team of scientists led by San Diego State University (San Diego, CA, USA) has shown how a well-known passive micromixer design (staggered herringbone mixer - SHM) can be optimized to induce maximum chaotic advection within antibody-coated channels of dimensions appropriate for the capture of cancer cell clusters. The device’s principle design configuration is called: Single-Walled Staggered Herringbone (SWaSH).
The design of the device makes use of 32 channels, each of 200 μm width and 100 μm spacing, which will increase the available chip surface to cross-sectional area by approximately 1.4-fold. Numerous simulations were performed by varying different properties of the HB pattern, such as channel configuration, and flow velocities to optimize for our deterministic factor cell-to-surface interactions. The Cy5-labeled streptavidin was utilized to visualize the cross-linked and functionalized alginate hydrogel coating within the micro channels. Images were captured using a fluorescence Zeiss 200M microscope.
Peter Teriete, PhD, an assistant professor and co-author of the study, said, “Our device's channel design had to generate microfluidic flow characteristics suitable to facilitate cell capture via antibodies within the coated channels. So we introduced microfeatures, herringbone recesses, to produce the desired functionality. We also developed a unique alginate hydrogel coating that can be readily arrayed with antibodies or other biomolecules. By connecting bioengineering with materials science and basic cancer biology, we were able to develop a device and prove that it performs as desired.” The study was published on June 18, 2019, in the journal AIP Advances.
Related Links:
San Diego State University














