Hormone Receptor Antibody Reagents Assessed for Breast Cancer
By LabMedica International staff writers
Posted on 08 Aug 2017
Immunohistochemical analysis of estrogen receptor (ER) and progesterone receptor (PgR) expression in breast cancer is the current standard of care and directly determines therapy.Posted on 08 Aug 2017
Estrogen receptor–expressing breast cancers tend to have better prognosis than ER-negative breast cancers and even more importantly, patients with strongly ER-positive tumors derive substantial benefit from therapies inhibiting estrogen signaling.
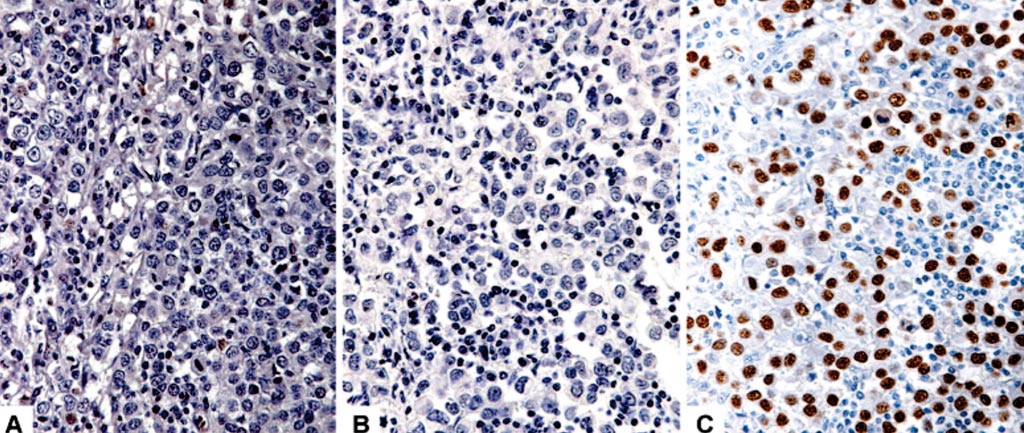
Image: Breast cancer: immunohistochemical comparison of three estrogen receptor antibodies: A through C, 1D5 and 6F11 show negativity, whereas SP1 shows positivity (Photo courtesy of Stanford University Medical Center).
Scientists at the Stanford University Medical Center (Stanford, CA, USA) compared the performance of different antibody reagents for ER and PgR immunohistochemical analysis by using College of American Pathologists (CAP) proficiency testing data. The team retrospectively analyzed tissue microarrays of ten 2-mm cores per slide and analyzed survey data from 80 ER and 80 PgR cores by antibody clone from more than 1,200 laboratories. The laboratories were instructed to stain the provided slides by using their clinically validated protocols (assuming a negative control is run and is appropriately negative) and score them according to the ASCO/CAP guidelines.
The team found that laboratories used the following ER antibodies: SP1 (72%), 6F11 (17%), 1D5 (3%), and the PgR antibodies 1E2 (61%), 16 (12%), PgR-636 (13%), PgR-1294 (8%) in 2015. While 63 of 80 ER cores (79%) were scored similarly using each of the three antibodies, there were significant differences for others, with SP1 yielding more positive interpretations. Four cores were scored as ER negative by more than half of the laboratories using 1D5 or 6F11, while SP1 produced positive results in more than 70% of laboratories using that antibody. Despite the greater variety of PgR antibody reagents and greater PgR tumor heterogeneity, 61 of 80 cores (76%) were scored similarly across the four PgR antibodies.
The authors concluded that analysis of proficiency testing data provides useful comparative data regarding analytic variables in ER and PgR testing. They highlighted the striking differences in ER results by antibody clone, with SP1 yielding more positive results than either 6F11 or 1D5. Accurate ER and PgR testing in breast cancer is crucial for appropriate treatment. The study was published on July 17, 2017, in the journal Archives of Pathology & Laboratory Medicine.
Related Links:
Stanford University Medical Center