Blood Test for Colorectal Cancer Recurrence Proves Highly Sensitive
By LabMedica International staff writers
Posted on 27 Oct 2016
When diagnosed early, before cancer has spread, the relative five-year survival rate for colorectal cancer (CRC) is 90%, but only approximately four out of 10 CRC cases are detected early.Posted on 27 Oct 2016
Regular blood testing for carcinoembryonic antigen (CEA), every three to six months is currently the only blood test recommended for routine monitoring of CRC, and while CEA measurement is the most sensitive simple test to aid in the monitoring of CRC, its sensitivity depends on what blood level, or changes in it, are chosen for positivity.
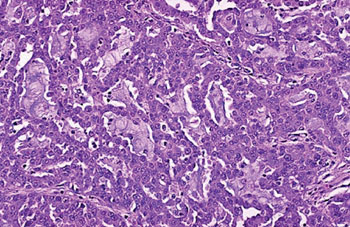
Image: A histopathology of a moderately differentiated adenocarcinoma of colon showing a glandular configuration, but the glands are irregular and very crowded. Many of them have lumens containing bluish mucin (Photo courtesy of Raul Gonzalez MD).
Scientists at the Flinders University of South Australia (Bedford Park, Australia) collected blood samples from any adults 18 years of age or older who were recently diagnosed but without residual macroscopic disease after initial treatment for CRC (AJCC stages I–IV) or who were already undergoing surveillance monitoring for CRC recurrence at Flinders Medical Centre during the period of 24 months from November 2013.
The concentration of CEA was determined using the LIAISON CEA test (DiaSorin S.p.A., Saluggia, Italy). A sample with CEA levels of 5 ng/mL or above was deemed positive, as commonly applied. The presence of methylated branched-chain amino acid transaminase 1 (BCAT1) and ikaros family zinc finger protein 1 (IKZF1) DNA in 3.9 mL plasma was determined as previously reported. The goal of the study, which incorporated clinic visits, blood sampling, and diagnostic imaging, was to compare the sensitivity and specificity of the BCAT1/IKZF1 blood test (Clinical Genomics, Bridgewater, NJ, USA) to CEA, when applied on a single occasion, for detection of recurrent CRC in patients undergoing surveillance. True- and false-positive rates for radiologically or histopathologically confirmed recurrence were compared and absolute sensitivity and specificity derived from these.
Of the 122 participants evaluated, 28 had recurrence and 94 had no clinically detectable disease. Among those with recurrent disease, 19/28 (67.9%) were positive for methylated BCAT1/IKZF1 while only 9/28 (32.1%) were positive for CEA, of greater than 5 ng/mL, representing a significant two-fold detection improvement with the two-gene test. Among the 94 patients without clinically detectable recurrence there was no significant difference in the percentage positive for methylated BCAT1/IKZF1 compared to CEA. In this study population, sensitivity estimates of the methylated BCAT1/IKZF1 test were 75% and 66.7% for local and distant recurrence, respectively, compared with 50% and 29.2% for CEA. Nine patients were positive for both tests, while the two-gene test detected an additional 10 cases that CEA failed to detect in the blood sample collected closest to the time of radiologic assessment for recurrence.
Lawrence LaPointe, PhD, President and CEO of Clinical Genomics said, “These data demonstrate that a blood-based virculating tumor DNA (ctDNA) test for methylated BCAT1/IKZF1 routinely detects recurrence that CEA testing misses. We believe our two-gene test has the potential to fill an urgent and unmet clinical need, and are committed to advancing its clinical development as a new tool for improving patient outcomes.” The study was published on October 11, 2016, in the journal Cancer Medicine.
Related Links:
Flinders University of South Australia
DiaSorin
Clinical Genomics














