Novel Device Enables Rapid Identification of Brain Cancer Type
By LabMedica International staff writers
Posted on 20 Oct 2016
A device has been developed for quick, accurate identification of a mutation strongly associated with a cancer that affects the central nervous system, potentially enabling accurate removal of the entire tumor during an operation.Posted on 20 Oct 2016
Gliomas are tumors occurring in the brain or spinal cord and are difficult to treat as they lack a clear edge, which complicates full surgical removal, which leads to high levels of recurrence and mortality. A particular mutation that is very common in gliomas has been identified, but is rare in other cancers and in normal tissue.
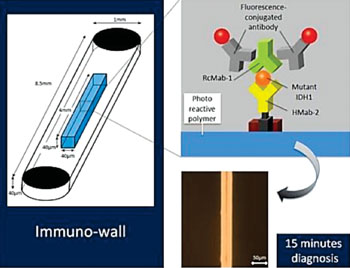
Image: Immuno-wall chips with the photo reactive polymer in the center of the 40 microchannels are made with a biotinylated anti-R132H-IDH1 antibody (HMab-2), an anti-wild-type IDH1 antibody (RcMab-1), and fluorescent antibodies. It shows sensitive and specific fluorescence from mutant IDH1 (Photo courtesy of Nagoya University).
Scientists at the Nagoya University (Japan) collected fresh tumor samples, 5–10 mm in diameter intraoperatively from 10 patients whose tumors were resected in 2015. The location of each sample was recorded stereotactically in an intraoperative navigation system. Each tumor tissue was dissected into three pieces for the immuno-wall assay, immunohistochemistry, and DNA sequencing.
The scientists used various techniques including cell lines expressing mutated or wild type isocitrate dehydrogenase 1 (IDH1), protein lysates, Western blotting, direct sequencing for IDH1 mutation were carried out using an ABI 3100 Genetic Analyzer (Applied Biosystems, Forest City, CA, USA). Detection and calculation of the frequency of the mutant allele was performed using pyrosequencing technology (Pyrosequencing AB, Uppsala, Sweden). An immuno-wall assay was developed using immuno-wall chips with 40 microchannels (1 mm width, 40 μm height and 8.5 mm length each) in a cyclic-olefin-polymer substrate were constructed using photolithography.
The device features a chip with an attached highly specific antibody, which binds to the protein produced by the gene in which the mutation has occurred. When a sample containing the mutated protein is added to the device, the protein binds to the antibody, which is then specifically detected by a source of fluorescence. In contrast, if the sample is from normal tissue without this mutation, or is from a tumor other than a glioma, no fluorescence occurs. The small sample size required for the device reduces the invasiveness of sample harvesting. In fact the process takes only 15 minutes, enabling completion during an operation. The immuno-wall could markedly increase success of glioma treatment by rapidly providing data to inform the course of the operation and tissue to remove.
The authors commented that the immuno-wall determines whether a sample is positive for a specific mutation in the isocitrate dehydrogenase 1 gene, which is present in around 70%-80% of grade II and III gliomas. Our results for a range of cancerous cell lines and actual tumor samples both positive and negative for this mutation were very promising. The device was proven highly accurate, as confirmed by complete sequencing of the gene in question in each sample. The study was published on October 4, 2016, in the journal Science and Technology of Advanced Materials.
Related Links:
Nagoya University
Applied Biosystems
Pyrosequencing AB