Deubiquitinating Enzyme A Biomarker for Endometrial Cancer Recurrence
By LabMedica International staff writers
Posted on 31 May 2016
Cancer researchers have found that expression of the deubiquitinating enzyme USP14 (Ubiquitin-specific protease 14) is elevated in endometrial adenocarcinoma and could serve as a biomarker to identify patients at risk for recurrence of the disease.Posted on 31 May 2016
Most endometrial cancer cases are diagnosed at an early stage, and patients have a good chance of recovery. However, a subset of patients with early stage and low-grade disease experience recurrence for reasons that remains unclear. Recurrence is often accompanied by chemoresistance and high mortality.
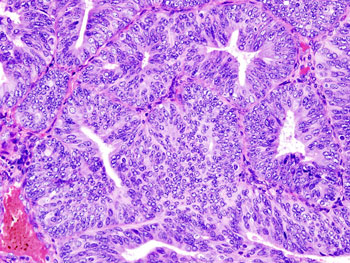
Image: A histologic view of an endometrial adenocarcinoma showing many abnormal nuclei (Photo courtesy of Wikimedia Commons).
Investigators at the University of Minnesota (Minneapolis, USA) have found that the chemoresistance may be linked to the expression of the deubiquitinating enzyme (DUB) USP14. DUBs are key components of the ubiquitin-dependent protein degradation pathway and act as master regulators in a number of metabolic processes including cell growth, differentiation, and apoptosis. They have been shown to be upregulated in a number of human cancers, and their aberrant activity has been linked to cancer progression, initiation, and onset of chemoresistance.
The investigators found that USP14 was expressed along with the marker of proliferation Ki67 in endometrial cancer cells in situ. Furthermore, pharmacological targeting of USP14 with the [U.S.] Food and Drug Administration approved small-molecule inhibitor VLX1570, decreased cell viability in chemotherapy resistant endometrial cancer cells with a mechanism consistent with cell cycle arrest and caspase-3 mediated apoptosis.
"We have discovered that women with high levels of USP14 are seven time more likely to recur than women with low levels of it," said senior author Dr. Martina Bazzaro, assistant professor of obstetrics, gynecology and women's health at the University of Minnesota. "Knowing a patient's status with regards to USP14 positivity could make a tremendous difference in terms of how a patient is treated and ultimately save her life."
"Our next step is a clinical trial. Patients with low risk endometrial cancer will be given the diagnostic exam, utilizing USP14 to gauge the levels of the cancer," said Dr. Bazzaro. "Those with high amounts - a positive test - will be treated more aggressively than current treatments to help prevent potential recurrence. Knowing more about their individual cancers can help us as clinicians to tailor a care plan specifically for them."
The study was published in the April 18, 2016, online edition of the journal Oncotarget.
Related Links:
University of Minnesota














