Screening Portfolio Protects Patients from Cervical Cancer and Overtreatment
By LabMedica International staff writers
Posted on 21 Oct 2015
A new portfolio of clinically validated genetic and pathology tests for human papillomavirus (HPV) cervical cancer (CC) screening and diagnosis helps identify women at high risk of developing this highly preventable disease, which is nevertheless still classified as the 4th most frequent cancer in women—530,000 new cases annually, of which about half the patients die.Posted on 21 Oct 2015
The new Cervical Cancer Screening Portfolio from Roche (Basel, Switzerland) has integrated testing to support laboratories and gynecologists in providing optimal patient care, and is unique in that it helps screen, diagnose, and manage patients. Most CC cases occur in countries that have not yet established systematic screening. Introduction of systematic Papanicolaou test (Pap smear) screening for precancerous lesions in many countries has helped reduce the number of women affected by or who die from CC by at least 80%. Although the incidence of the disease has been greatly reduced through the Pap test, data has shown its limitations, as a consequence up to a third of CCs occur in women with normal Pap results.
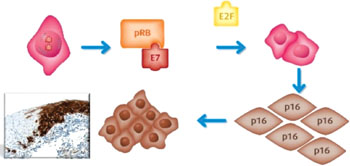
Image: In Roche’s Cervical Cancer Screening Portfolio, the “CINtec p16 Histology” immunohistochemistry assay detects p16-INK4a in cervical biopsy preparations. The figure illustrates that in cells with transforming HPV infections, HPV viral oncoprotein E7 impairs the function of pRB, disrupting its ability to bind to E2F. This leads to deregulated cell proliferation, genetic instability, and p16 over-expression detectible by immunohistochemistry staining (Image courtesy of Ventana, of Roche).
CC caused by persistent HPV infection can take over 10–15 years to develop into full blown disease. Though most of the over 100 HPV types are cleared naturally by the body, high-risk HPV types cause 99% of CCs, 70% by two specific genotypes–HPV-16 and -18. CC screening is in transition and technologies have advanced greatly: innovative molecular testing based on HPV DNA 16/18 genotyping, as well as immunocytological and immunohistological staining to detect p16-INK4a and Ki-67, have the ability to distinguish women with the greatest risk, women who need immediate management due to existence of lesions, and women who can safely return to routine screening. This means preventative measures can be started without delay, potentially saving lives by protecting women from unnecessary burden of cancer and its treatments.
Preventive practice is evolving in parallel with technological advancement. In the current US screening guidelines, co-testing for HPV in addition to Pap test is now preferred over Pap test alone for women aged 30–65. The recommendation is based on evidence that, compared to Pap cytology alone, adding the HPV test increases detection rate (for cervical cancer and its precursors) and reduces the rate of invasive CC. HPV test integration into primary screening will help protect more women. Following the 2014 US Food and Drug Administration (FDA) approval of Roche’s cobas HPV Test as a standalone primary screening test, two professional societies have now issued interim guidance on the use of HPV primary screening in the US. These changes reflect increasing adoption of HPV primary screening guidelines worldwide.
Cobas HPV Test is the only FDA-approved and CE-marked test for first-line primary HPV screening for identification of at-risk women. From one sample in a single test, Cobas HPV simultaneously determines a pooled result for 12 high-risk genotypes, and provides separate results for the two highest risk genotypes: HPV-16 and HPV-18. This differential allows a more reliable determination of an individual’s risk.
CINtec PLUS Cytology, an immuno-cytochemistry assay for identification of a high-grade cervical lesion (CIN2+), is the only test that uses dual-biomarker technology to simultaneously detect p16-INK4a and Ki-67 to provide a strong indicator of the presence of transforming HPV infection. The test is used as a triage tool to identify which women may benefit most from colposcopy.
CINtec p16 Histology, an immuno-histochemistry assay, detects the p16-INK4a biomarker in cervical biopsy preparations. Used in conjunction with Hematoxylin and Eosin (H&E) stained preparations, CINtec p16 Histology test allows pathologists to accurately and expediently diagnose and confirm a suspected high-grade cervical lesion, and so deliver definitive results that help clinicians make more informed decisions about patient follow-up.
Clinical validation of Roche’s cervical cancer screening portfolio tests
cobas HPV test: A landmark trial, in over 47,000 women aged ≥21 years receiving routine CC screening, clinically validated the cobas HPV Test in all relevant populations: ASC-US cytology, HPV and Pap co-testing and primary screening. This trial was also the largest prospective CC screening trial to evaluate the clinical use of HPV DNA testing with 16/18 genotyping. Published under the name ATHENA (Addressing THE Need for Advanced HPV diagnostics) in multiple reports in leading scientific journals, the trial is the largest pivotal HPV study ever to be conducted to date. In addition to ATHENA, other studies have been published, including two successful European validations of the test under the widely accepted European HPV testing criteria. Also, cobas HPV Test was approved in April 2011 by the FDA for the workup of equivocal Pap cytologies (ASC-US triage) and HPV and Pap co-screening on the strength of the ATHENA data. FDA approval for stand-alone primary HPV screening of women aged 25 and above followed in April 2014.
CINtec PLUS Cytology and CINtec p16 Histology: Both tests have extensive clinical evidence to support their application in screening and diagnosis.
When used as a triage for HPV testing, CINtec PLUS Cytology has demonstrated high sensitivity and specificity in detecting ≥CIN2 lesions, thus avoiding unnecessary colposcopy. Also, in patients aged 30 and over, with a normal Pap cytology result but positive for HPV, CINtec PLUS Cytology can add benefit through detection of underlying ≥CIN2.
In diagnosis of cervical pre-cancer, the adjunctive use of the CINtec p16 Histology test increased sensitivity for the detection of CIN3 by 11% compared with H&E staining alone. The false negative rate was reduced by 45% due to the significantly increased accuracy when using the CINtec p16 Histology in conjunction with H&E. The test can also identify lesions previously missed using H&E alone. The “LAST Working Group” recommends use of the p16-INK4a biomarker as an adjunct to H&E diagnosis as the only biomarker with sufficient evidence, recommendations that have subsequently been adopted into World Health Organisation (WHO) guidelines.
Related Links:
Roche
Roche Cervical Cancer Prevention Portfolio














