Likelihood of Lymphoma Recurrence Can Be Predicted by Monitoring Blood Levels of Circulating Tumor DNA
By LabMedica International staff writers
Posted on 15 Apr 2015
A recent paper described the measurement of circulating tumor DNA (ctDNA) to diagnose patients with diffuse large B-cell lymphoma (DLBCL) and to predict their likely response to treatment.Posted on 15 Apr 2015
DLBCL is a treatable form of cancer, but when treatment fails, outcome is poor. Although imaging can help to identify patients at risk of treatment failure, they are often imprecise, and radiation exposure is a potential health risk. Investigators at the [US] National Cancer Institute (Bethesda, MD, USA) examined the possibility that ctDNA encoding the clonal immunoglobulin gene sequence could be detected in the serum of patients with DLBCL and used to predict clinical disease recurrence after chemotherapeutic treatment.
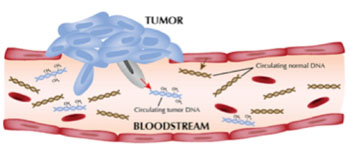
Image: Measurement of circulating tumor DNA (ctDNA) can be used to diagnose patients with diffuse large B-cell lymphoma (DLBCL) and to predict their likely response to treatment (Photo courtesy of National Cancer Institute).
To this end the investigators obtained serial serum samples and concurrent CT scans at specified times during most treatment cycles and up to five years of follow-up from 126 patients who had no evidence of indolent lymphoma and were previously untreated. The patients were then assigned to one of three treatment protocols between May 1993, and June 2013.
Serum samples were tested retroactively using next-generation DNA sequencing techniques to analyze cell-free circulating tumor DNA. Results revealed that among the 107 patients who achieved complete remission following treatment, those who developed detectable ctDNA during surveillance were over 200 times more likely to have their disease progress than those who did not have detectable ctDNA. The investigators also found that measuring ctDNA enabled the detection of cancer recurrence a median of 3.4 months before clinical evidence of disease. Furthermore, following an approach known as interim monitoring, ctDNA analysis was able to predict which patients would not respond to therapy as early as their second cycle of treatment.
“Even with frequent CT imaging, administered for a median of 11 times per patient in our study, early disease detection was suboptimal. Indeed, a recent study suggested that surveillance CT scans might be no better than an up-to-date patient history and physical exams, supporting the need for more effective monitoring technologies,” said senior author Dr. Wyndham Wilson, senior investigator in the lymphoid malignancies branch of the National Cancer Institute. . “Interim ctDNA is therefore a promising biomarker to identify patients at high risk of not responding to treatment for their disease.”
Related Links:
[US] National Cancer Institute











.jpg)