New Partnership Designed to Automate Dystrophin Measurement
By LabMedica International staff writers
Posted on 01 Sep 2014
A developer of innovative ribonucleic acid (RNA)-based therapeutics, Sarepta Therapeutics, Inc. ( Cambridge, MA, USA), and a leading tissue-based companion diagnostics firm, Flagship Biosciences LLC (Boulder, CO, USA) announced a multiyear, multiproduct partnership.Posted on 01 Sep 2014
The partnership is for the development of automated quantitative endpoint measurements in muscular dystrophy to support the advancement of Sarepta's Duchenne muscular dystrophy (DMD) drug pipeline, including its lead candidate, eteplirsen. DMD is caused by the absence of functional dystrophin in affected patients' muscle tissue.
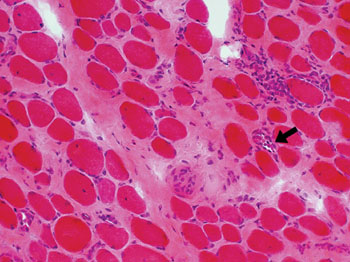
Image: Histopathology of increased endomysial connective tissue from a patient with Duchenne Muscular Dystrophy. Necrotic fibers arrowed (Photo courtesy of Washington University in St. Louis).
Dystrophin protein level is a fundamental biomarker used to assess therapies that aim to produce and restore the expression of dystrophin, such as exon-skipping therapies like eteplirsen. In order to optimally and efficiently evaluate therapeutic efficacy in patients, the next generation of protocols are being developed to digitally automate and standardize dystrophin measurement in tissue biopsies to speed the process while ensuring consistency. The establishment of these new standardized methods for automated quantitation is being enabled though the proprietary image analysis platform and digital pathology capabilities developed by Flagship Bioscience.
DMD is an X-linked rare degenerative neuromuscular disorder causing severe progressive muscle loss and premature death. A devastating and incurable muscle-wasting disease, DMD is associated with specific errors in the gene that codes for dystrophin, a protein that plays a key structural role in muscle fiber function. Progressive muscle weakness in the lower limbs spreads to the arms, neck and other areas. Eventually, increasing difficulty in breathing due to respiratory muscle dysfunction requires ventilation support, and cardiac dysfunction can lead to heart failure. The condition is universally fatal, and death usually occurs before the age of 30.
G. David Young, DVM, DACVP, DABT, Director of Pathology at Flagship, said, “Flagship Biosciences has developed tools and expertise in quantitative pathology, image analysis, and tissue-based assays that are well-suited for use in a regulated environment. It's exciting to work with a partner like Sarepta to design and implement an integrated fit-for-purpose assay and automated quantitative interpretation approach that accelerates the development of drugs for unmet needs such as eteplirsen for the treatment of DMD."
Related Links:
Sarepta Therapeutics
Flagship Biosciences