At-Home Blood Tests Accurately Detect Key Alzheimer's Biomarkers
Posted on 08 Jan 2026
Diagnosing Alzheimer’s disease typically relies on brain scans or spinal fluid tests, which are invasive, costly, and difficult to access outside specialist clinics. These barriers have limited large-scale research and excluded many populations from participating in studies, particularly in remote or underserved regions. A new study now shows that Alzheimer’s disease biomarkers can be accurately measured from finger-prick blood samples collected at home and mailed to laboratories without refrigeration.
Under the DROP-AD project, a study led by Banner Health (Phoenix, AZ, US) in collaboration with the University of Exeter Medical School (Exeter, UK), evaluated a dried blood spot method using a few drops of blood from a fingertip placed on a collection card. This approach removes the need for venipuncture, cold storage, or specialized clinical infrastructure.
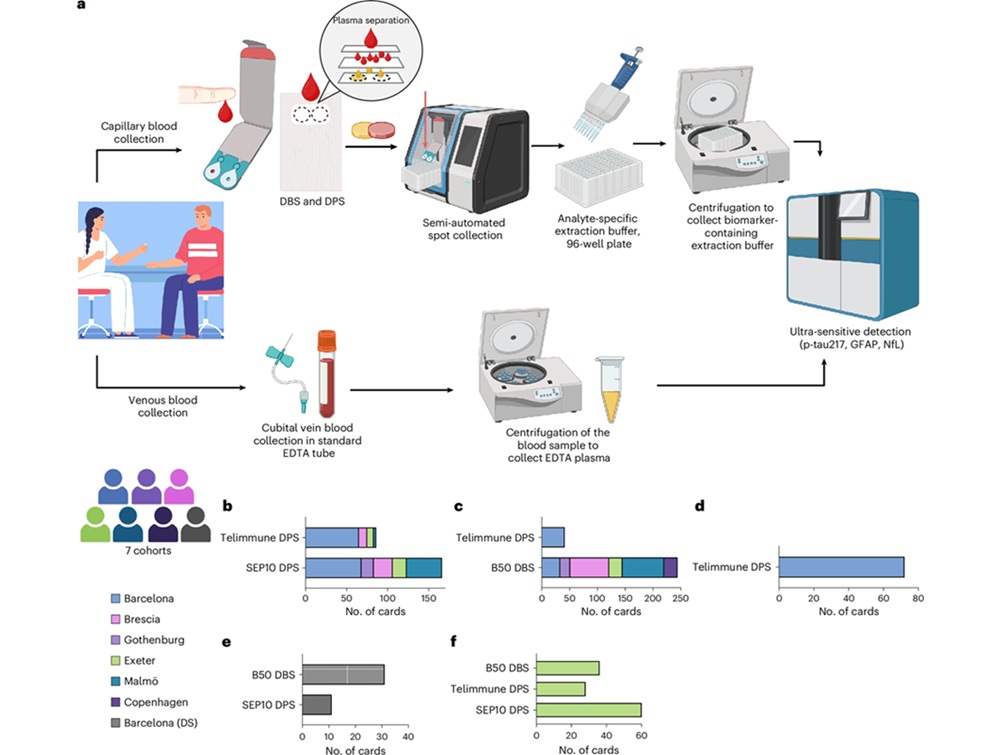
The international study enrolled 337 participants across seven European medical centers, including sites in Gothenburg and Exeter. Researchers measured Alzheimer’s-related biomarkers from dried finger-prick blood samples and compared the results with standard venous blood tests and cerebrospinal fluid markers. The University of Exeter site also evaluated self-collection, with participants successfully collecting samples at home after written instructions and demonstrations.
Levels of phosphorylated tau (p-tau217) measured from finger-prick samples closely matched results from conventional blood tests and identified Alzheimer’s-related spinal fluid changes with 86% accuracy. Two additional biomarkers, glial fibrillary acidic protein (GFAP) and neurofilament light (NfL), were also reliably detected and showed strong agreement with traditional methods. The findings, published in Nature Medicine, mark the first large-scale validation of this remote testing approach.
The technique enables remote participation in Alzheimer’s research, supports large-scale population studies, and may improve clinical trial recruitment and monitoring. It could also expand research access for underrepresented communities, people in low-resource settings, and groups at higher risk, such as individuals with Down syndrome. While not yet ready for clinical use, researchers believe the method could eventually support earlier identification of people who may benefit from further diagnostic testing or emerging therapies.
“This breakthrough could fundamentally change how we conduct Alzheimer’s research by proving that the same biomarkers doctors use to detect Alzheimer’s pathology can be measured from a simple finger prick collected at home,” said Nicholas Ashton, PhD, lead investigator of the study. “We’re opening doors to research that was previously impossible, although further validation is still needed before clinical use.”
Related Links:
Banner Health
University of Exeter Medical School














